Allosteric modulation of Ras-GTP is linked to signal transduction through RAF kinase
- PMID: 21098031
- PMCID: PMC3030338
- DOI: 10.1074/jbc.M110.193854
Allosteric modulation of Ras-GTP is linked to signal transduction through RAF kinase
Abstract
Ras is a key signal transduction protein in the cell. Mutants of Gly(12) and Gln(61) impair GTPase activity and are found prominently in cancers. In wild type Ras-GTP, an allosteric switch promotes disorder to order transition in switch II, placing Gln(61) in the active site. We show that the "on" and "off" conformations of the allosteric switch can also be attained in RasG12V and RasQ61L. Although both mutants have similarly impaired active sites in the on state, RasQ61L stabilizes an anti-catalytic conformation of switch II in the off state of the allosteric switch when bound to Raf. This translates into more potent activation of the MAPK pathway involving Ras, Raf kinase, MEK, and ERK (Ras/Raf/MEK/ERK) in cells transfected with RasQ61L relative to RasG12V. This differential is not observed in the Raf-independent pathway involving Ras, phosphoinositide 3-kinase (PI3K), and Akt (Ras/PI3K/Akt). Using a combination of structural analysis, hydrolysis rates, and experiments in NIH-3T3 cells, we link the allosteric switch to the control of signaling in the Ras/Raf/MEK/ERK pathway, supporting a GTPase-activating protein-independent model for duration of the Ras-Raf complex.
Figures


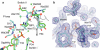


References
-
- Cox A. D., Der C. J. (2003) Oncogene 22, 8999–9006 - PubMed
-
- Thapar R., Williams J. G., Campbell S. L. (2004) J. Mol. Biol. 343, 1391–1408 - PubMed
-
- Pacold M. E., Suire S., Perisic O., Lara-Gonzalez S., Davis C. T., Walker E. H., Hawkins P. T., Stephens L., Eccleston J. F., Williams R. L. (2000) Cell 103, 931–943 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

