Hyperosmotic stress-induced ATF-2 activation through Polo-like kinase 3 in human corneal epithelial cells
- PMID: 21098032
- PMCID: PMC3023491
- DOI: 10.1074/jbc.M110.166009
Hyperosmotic stress-induced ATF-2 activation through Polo-like kinase 3 in human corneal epithelial cells
Abstract
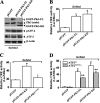
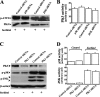
Elevated extracellular solute concentration (hyperosmotic stress) perturbs cell function and stimulates cell responses by evoking MAPK cascades and activating AP-1 transcription complex resulting in alterations of gene expression, cell cycle arrest, and apoptosis. The results presented here demonstrate that hyperosmotic stress elicited increases in ATF-2 phosphorylation through a novel Polo-like kinase 3 (Plk3) pathway in human corneal epithelial (HCE) cells. We found in hyperosmotic stress-induced HCE cells that Plk3 transferred to the nuclear compartment and was colocalized with ATF-2 in nuclei. Kinase activity of Plk3 was significantly activated by hyperosmotic stimulation. Further downstream, active Plk3 phosphorylated ATF-2 at the Thr-71 site in vivo and in vitro. Overexpression of Plk3 and its mutants enhanced hyperosmotic stress-induced ATF-2 phosphorylation. In contrast, suppression of Plk3 by knocking down Plk3 mRNA effectively diminished the effect of hyperosmotic stress-induced ATF-2 phosphorylation. The effect of hyperosmotic stress-induced activation of Plk3 on ATF-2 transcription factor function was also examined in CRE reporter-overexpressed HCE cells. Our results for the first time reveal that hyperosmotic stress can activate the Plk3 signaling pathway that subsequently regulates the AP-1 complex by directly phosphorylating ATF-2 independent from the effects of JNK and p38 activation.
Figures
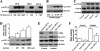
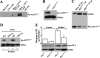



Similar articles
-
Osmotic stress-induced phosphorylation of H2AX by polo-like kinase 3 affects cell cycle progression in human corneal epithelial cells.J Biol Chem. 2014 Oct 24;289(43):29827-35. doi: 10.1074/jbc.M114.597161. Epub 2014 Sep 8. J Biol Chem. 2014. PMID: 25202016 Free PMC article.
-
Hyperosmotic stress-induced corneal epithelial cell death through activation of Polo-like kinase 3 and c-Jun.Invest Ophthalmol Vis Sci. 2011 May 16;52(6):3200-6. doi: 10.1167/iovs.10-6485. Invest Ophthalmol Vis Sci. 2011. PMID: 21296815 Free PMC article.
-
Activation of Polo-like kinase 3 by hypoxic stresses.J Biol Chem. 2008 Sep 19;283(38):25928-35. doi: 10.1074/jbc.M801326200. Epub 2008 Jul 23. J Biol Chem. 2008. PMID: 18650425 Free PMC article.
-
Nuclear export of the stress-activated protein kinase p38 mediated by its substrate MAPKAP kinase-2.Curr Biol. 1998 Sep 24;8(19):1049-57. doi: 10.1016/s0960-9822(98)70442-7. Curr Biol. 1998. PMID: 9768359 Review.
-
Inheritance and memory of stress-induced epigenome change: roles played by the ATF-2 family of transcription factors.Genes Cells. 2012 Apr;17(4):249-63. doi: 10.1111/j.1365-2443.2012.01587.x. Epub 2012 Mar 1. Genes Cells. 2012. PMID: 22380515 Free PMC article. Review.
Cited by
-
Phosphorylation of PLK3 Is Controlled by Protein Phosphatase 6.Cells. 2020 Jun 20;9(6):1506. doi: 10.3390/cells9061506. Cells. 2020. PMID: 32575753 Free PMC article.
-
Differential effects of hepatocyte growth factor and keratinocyte growth factor on corneal epithelial cell cycle protein expression, cell survival, and growth.Mol Vis. 2014 Jan 6;20:24-37. eCollection 2014. Mol Vis. 2014. PMID: 24426773 Free PMC article.
-
Nuclear receptor LRH-1/NR5A2 is required and targetable for liver endoplasmic reticulum stress resolution.Elife. 2014 Apr 15;3:e01694. doi: 10.7554/eLife.01694. Elife. 2014. PMID: 24737860 Free PMC article.
-
The unfolded protein response and the phosphorylations of activating transcription factor 2 in the trans-activation of il23a promoter produced by β-glucans.J Biol Chem. 2014 Aug 15;289(33):22942-22957. doi: 10.1074/jbc.M113.522656. Epub 2014 Jun 30. J Biol Chem. 2014. PMID: 24982422 Free PMC article.
-
ATF-2 immunoreactivity in post-mitotic and terminally differentiated human odontoblasts.Med Mol Morphol. 2015 Sep;48(3):164-8. doi: 10.1007/s00795-014-0092-x. Epub 2014 Nov 23. Med Mol Morphol. 2015. PMID: 25417007
References
-
- Lang F., Busch G. L., Ritter M., Völkl H., Waldegger S., Gulbins E., Häussinger D. (1998) Physiol. Rev. 78, 247–306 - PubMed
-
- Deutsch C., Slater L., Goldstein P. (1982) Biochim. Biophys. Acta 721, 262–267 - PubMed
-
- Burg M. B., Ferraris J. D., Dmitrieva N. I. (2007) Physiol. Rev. 87, 1441–1474 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

