Syk-dependent phosphorylation of CLEC-2: a novel mechanism of hem-immunoreceptor tyrosine-based activation motif signaling
- PMID: 21098033
- PMCID: PMC3039337
- DOI: 10.1074/jbc.M110.167502
Syk-dependent phosphorylation of CLEC-2: a novel mechanism of hem-immunoreceptor tyrosine-based activation motif signaling
Abstract
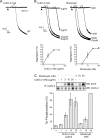
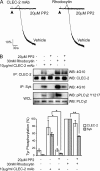
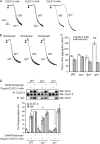
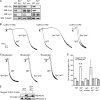
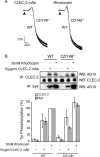
The C-type lectin-like receptor CLEC-2 signals via phosphorylation of a single cytoplasmic YXXL sequence known as a hem-immunoreceptor tyrosine-based activation motif (hemITAM). In this study, we show that phosphorylation of CLEC-2 by the snake toxin rhodocytin is abolished in the absence of the tyrosine kinase Syk but is not altered in the absence of the major platelet Src family kinases, Fyn, Lyn, and Src, or the tyrosine phosphatase CD148, which regulates the basal activity of Src family kinases. Further, phosphorylation of CLEC-2 by rhodocytin is not altered in the presence of the Src family kinase inhibitor PP2, even though PLCγ2 phosphorylation and platelet activation are abolished. A similar dependence of phosphorylation of CLEC-2 on Syk is also seen in response to stimulation by an IgG mAb to CLEC-2, although interestingly CLEC-2 phosphorylation is also reduced in the absence of Lyn. These results provide the first definitive evidence that Syk mediates phosphorylation of the CLEC-2 hemITAM receptor with Src family kinases playing a critical role further downstream through the regulation of Syk and other effector proteins, providing a new paradigm in signaling by YXXL-containing receptors.
Figures


Similar articles
-
A novel Syk-dependent mechanism of platelet activation by the C-type lectin receptor CLEC-2.Blood. 2006 Jan 15;107(2):542-9. doi: 10.1182/blood-2005-05-1994. Epub 2005 Sep 20. Blood. 2006. PMID: 16174766
-
The C-type lectin receptors CLEC-2 and Dectin-1, but not DC-SIGN, signal via a novel YXXL-dependent signaling cascade.J Biol Chem. 2007 Apr 27;282(17):12397-409. doi: 10.1074/jbc.M609558200. Epub 2007 Mar 5. J Biol Chem. 2007. PMID: 17339324 Free PMC article.
-
Gq pathway regulates proximal C-type lectin-like receptor-2 (CLEC-2) signaling in platelets.J Biol Chem. 2017 Sep 1;292(35):14516-14531. doi: 10.1074/jbc.M117.791012. Epub 2017 Jul 13. J Biol Chem. 2017. PMID: 28705934 Free PMC article.
-
GPVI and CLEC-2 in hemostasis and vascular integrity.J Thromb Haemost. 2010 Jul;8(7):1456-67. doi: 10.1111/j.1538-7836.2010.03875.x. Epub 2010 Mar 25. J Thromb Haemost. 2010. PMID: 20345705 Review.
-
Thrombomodulation via CLEC-2 targeting.Curr Opin Pharmacol. 2009 Apr;9(2):90-5. doi: 10.1016/j.coph.2008.11.001. Epub 2008 Dec 16. Curr Opin Pharmacol. 2009. PMID: 19091630 Review.
Cited by
-
Distinct pathways regulate Syk protein activation downstream of immune tyrosine activation motif (ITAM) and hemITAM receptors in platelets.J Biol Chem. 2015 May 1;290(18):11557-68. doi: 10.1074/jbc.M114.629527. Epub 2015 Mar 12. J Biol Chem. 2015. PMID: 25767114 Free PMC article.
-
Clustering extent-dependent differential signaling by CLEC-2 receptors in platelets.Res Pract Thromb Haemost. 2022 May 6;6(3):e12710. doi: 10.1002/rth2.12710. eCollection 2022 Mar. Res Pract Thromb Haemost. 2022. PMID: 35573643 Free PMC article.
-
Establishment and maintenance of blood-lymph separation.Cell Mol Life Sci. 2019 May;76(10):1865-1876. doi: 10.1007/s00018-019-03042-3. Epub 2019 Feb 13. Cell Mol Life Sci. 2019. PMID: 30758642 Free PMC article. Review.
-
Modulation of NK cell function by genetically coupled C-type lectin-like receptor/ligand pairs encoded in the human natural killer gene complex.Front Immunol. 2013 Nov 7;4:362. doi: 10.3389/fimmu.2013.00362. Front Immunol. 2013. PMID: 24223577 Free PMC article. Review.
-
The Activating C-type Lectin-like Receptor NKp65 Signals through a Hemi-immunoreceptor Tyrosine-based Activation Motif (hemITAM) and Spleen Tyrosine Kinase (Syk).J Biol Chem. 2017 Feb 24;292(8):3213-3223. doi: 10.1074/jbc.M116.759977. Epub 2017 Jan 12. J Biol Chem. 2017. PMID: 28082678 Free PMC article.
References
-
- Suzuki-Inoue K., Fuller G. L., García A., Eble J. A., Pöhlmann S., Inoue O., Gartner T. K., Hughan S. C., Pearce A. C., Laing G. D., Theakston R. D., Schweighoffer E., Zitzmann N., Morita T., Tybulewicz V. L., Ozaki Y., Watson S. P. (2006) Blood 107, 542–549 - PubMed
-
- Colonna M., Samaridis J., Angman L. (2000) Eur. J. Immunol. 30, 697–704 - PubMed
-
- Sobanov Y., Bernreiter A., Derdak S., Mechtcheriakova D., Schweighofer B., Düchler M., Kalthoff F., Hofer E. (2001) Eur. J. Immunol. 31, 3493–3503 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

