Binding of albumin promotes bacterial survival at the epithelial surface
- PMID: 21098039
- PMCID: PMC3024741
- DOI: 10.1074/jbc.M110.148171
Binding of albumin promotes bacterial survival at the epithelial surface
Abstract
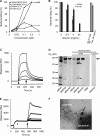
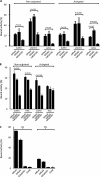
Human serum albumin (HSA) is the dominating protein in human plasma. Many bacterial species, especially streptococci, express surface proteins that bind HSA with high specificity and affinity, but the biological consequences of these protein-protein interactions are poorly understood. Group G streptococci (GGS), carrying the HSA-binding protein G, colonize the skin and the mucosa of the upper respiratory tract, mostly without causing disease. In the case of bacterial invasion, pro-inflammatory cytokines are released that activate the epithelium to produce antibacterial peptides, in particular the chemokine MIG/CXCL9. In addition, the inflammation causes capillary leakage and extravasation of HSA and other plasma proteins, environmental changes at the epithelial surface to which the bacteria need to respond. In this study, we found that GGS adsorbed HSA from both saliva and plasma via binding to protein G and that HSA bound to protein G bound and inactivated the antibacterial MIG/CXCL9 peptide. Another surface protein of GGS, FOG, was found to mediate adherence of the bacteria to pharyngeal epithelial cells through interaction with glycosaminoglycans. This adherence was not affected by activation of the epithelium with a combination of IFN-γ and TNF-α, leading to the production of MIG/CXCL9. However, at the activated epithelial surface, adherent GGS were protected against killing by MIG/CXCL9 through protein G-dependent HSA coating. The findings identify a previously unknown bacterial survival strategy that helps to explain the evolution of HSA-binding proteins among bacterial species of the normal human microbiota.
Figures


Similar articles
-
Protein FOG is a moderate inducer of MIG/CXCL9, and group G streptococci are more tolerant than group A streptococci to this chemokine's antibacterial effect.Microbiology (Reading). 2007 Nov;153(Pt 11):3800-3808. doi: 10.1099/mic.0.2007/009647-0. Microbiology (Reading). 2007. PMID: 17975089
-
M1 protein of Streptococcus pyogenes increases production of the antibacterial CXC chemokine MIG/CXCL9 in pharyngeal epithelial cells.Microb Pathog. 2007 Nov-Dec;43(5-6):224-33. doi: 10.1016/j.micpath.2007.06.007. Epub 2007 Aug 2. Microb Pathog. 2007. PMID: 17681739
-
The CXC chemokine MIG/CXCL9 is important in innate immunity against Streptococcus pyogenes.J Infect Dis. 2007 Mar 1;195(5):684-93. doi: 10.1086/510857. Epub 2007 Jan 18. J Infect Dis. 2007. PMID: 17262710
-
CXCL9 chemokine in ulcerative colitis.Clin Ter. 2018 Sep-Oct;169(5):e235-e241. doi: 10.7417/CT.2018.2085. Clin Ter. 2018. PMID: 30393811 Review.
-
Development, validation and implementation of an in vitro model for the study of metabolic and immune function in normal and inflamed human colonic epithelium.Dan Med J. 2015 Jan;62(1):B4973. Dan Med J. 2015. PMID: 25557335 Review.
Cited by
-
Streptococcus pyogenes in human plasma: adaptive mechanisms analyzed by mass spectrometry-based proteomics.J Biol Chem. 2012 Jan 6;287(2):1415-25. doi: 10.1074/jbc.M111.267674. Epub 2011 Nov 23. J Biol Chem. 2012. PMID: 22117078 Free PMC article.
-
The Antistaphylococcal Lysin, CF-301, Activates Key Host Factors in Human Blood To Potentiate Methicillin-Resistant Staphylococcus aureus Bacteriolysis.Antimicrob Agents Chemother. 2019 Mar 27;63(4):e02291-18. doi: 10.1128/AAC.02291-18. Print 2019 Apr. Antimicrob Agents Chemother. 2019. PMID: 30670427 Free PMC article.
-
A new model of endotracheal tube biofilm identifies combinations of matrix-degrading enzymes and antimicrobials able to eradicate biofilms of pathogens that cause ventilator-associated pneumonia.Microbiology (Reading). 2024 Aug;170(8):001480. doi: 10.1099/mic.0.001480. Microbiology (Reading). 2024. PMID: 39088248 Free PMC article.
-
Extracellular vesicles engineered to bind albumin demonstrate extended circulation time and lymph node accumulation in mouse models.J Extracell Vesicles. 2022 Jul;11(7):e12248. doi: 10.1002/jev2.12248. J Extracell Vesicles. 2022. PMID: 35879268 Free PMC article.
-
Albumin orchestrates a natural host defense mechanism against mucormycosis.Res Sq [Preprint]. 2024 Dec 3:rs.3.rs-5441197. doi: 10.21203/rs.3.rs-5441197/v1. Res Sq. 2024. PMID: 39678331 Free PMC article. Preprint.
References
-
- Sylvetsky N., Raveh D., Schlesinger Y., Rudensky B., Yinnon A. M. (2002) Am. J. Med. 112, 622–626 - PubMed
-
- Sansonetti P. J. (2004) Nat. Rev. Immunol. 4, 953–964 - PubMed
-
- Hyland K. A., Brennan R., Olmsted S. B., Rojas E., Murphy E., Wang B., Cleary P. P. (2009) FEMS Immunol. Med. Microbiol. 55, 422–431 - PubMed
-
- Raeder R. H., Barker-Merrill L., Lester T., Boyle M. D., Metzger D. W. (2000) J. Infect. Dis. 181, 639–645 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

