Gi/o-coupled receptors compete for signaling to adenylyl cyclase in SH-SY5Y cells and reduce opioid-mediated cAMP overshoot
- PMID: 21098043
- PMCID: PMC3061372
- DOI: 10.1124/mol.110.064816
Gi/o-coupled receptors compete for signaling to adenylyl cyclase in SH-SY5Y cells and reduce opioid-mediated cAMP overshoot
Abstract
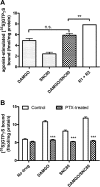
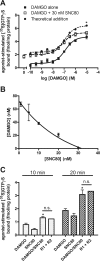
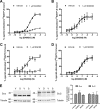
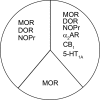
Organization of G protein-coupled receptors and cognate signaling partners at the plasma membrane has been proposed to occur via multiple mechanisms, including membrane microdomains, receptor oligomerization, and protein scaffolding. Here, we investigate the organization of six types of Gi/o-coupled receptors endogenously expressed in SH-SY5Y cells. The most abundant receptor in these cells was the μ-opioid receptor (MOR), the activation of which occluded acute inhibition of adenylyl cyclase (AC) by agonists to δ-opioid (DOR), nociceptin/orphanin FQ peptide (NOPr), α2-adrenergic (α2AR), cannabinoid 1, and serotonin 1A receptors. We further demonstrate that all receptor pairs share a common pool of AC. The MOR agonist [D-Ala2,N-Me-Phe4,Gly5-ol]-enkephalin (DAMGO) also occluded the ability of DOR agonist to stimulate G proteins. However, at lower agonist concentrations and at shorter incubation times when G proteins were not limiting, the relationship between MOR and DOR agonists was additive. The additive relationship was confirmed by isobolographic analysis. Long-term coadministration of MOR and DOR agonists caused cAMP overshoot that was not additive, suggesting that sensitization of AC mediated by these two receptors occurs by a common pathway. Furthermore, heterologous inhibition of AC by agonists to DOR, NOPr, and α2AR reduced the expression of cAMP overshoot in DAMGO-dependent cells. However, this cross-talk did not lead to heterologous tolerance. These results indicate that multiple receptors could be tethered into complexes with cognate signaling proteins and that access to shared AC by multiple receptor types may provide a means to prevent opioid withdrawal.
Figures
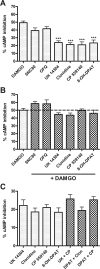
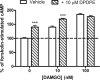
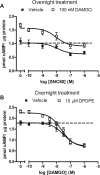
 ) in the presence or absence of the MOR agonist DAMGO (10 or 100 nM) to induce dependence. Withdrawal was precipitated with the opioid antagonist naloxone (100 μM) in the presence of 5 μM forskolin. Data are presented as mean ± S.E.M. (n = 4, in duplicate) of the percentage of forskolin-stimulated cAMP, where forskolin alone is 100% and is indicated by the dashed line. Overnight incubation with DPDPE produced overshoot on its own and enhanced the overshoot produced by 10 nM but not 100 nM DAMGO. ***, p < 0.001 compared with the vehicle with the same concentration of DAMGO by two-way ANOVA and Bonferroni's post test.
) in the presence or absence of the MOR agonist DAMGO (10 or 100 nM) to induce dependence. Withdrawal was precipitated with the opioid antagonist naloxone (100 μM) in the presence of 5 μM forskolin. Data are presented as mean ± S.E.M. (n = 4, in duplicate) of the percentage of forskolin-stimulated cAMP, where forskolin alone is 100% and is indicated by the dashed line. Overnight incubation with DPDPE produced overshoot on its own and enhanced the overshoot produced by 10 nM but not 100 nM DAMGO. ***, p < 0.001 compared with the vehicle with the same concentration of DAMGO by two-way ANOVA and Bonferroni's post test.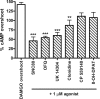
Similar articles
-
Mu and Delta opioid receptors activate the same G proteins in human neuroblastoma SH-SY5Y cells.Br J Pharmacol. 2002 Jan;135(1):217-25. doi: 10.1038/sj.bjp.0704430. Br J Pharmacol. 2002. PMID: 11786497 Free PMC article.
-
Opioid and cannabinoid receptors share a common pool of GTP-binding proteins in cotransfected cells, but not in cells which endogenously coexpress the receptors.Cell Mol Neurobiol. 2000 Jun;20(3):291-304. doi: 10.1023/a:1007058008477. Cell Mol Neurobiol. 2000. PMID: 10789829 Free PMC article.
-
Mediation of adenylyl cyclase sensitization by PTX-insensitive GalphaoA, Galphai1, Galphai2 or Galphai3.J Neurochem. 2006 Dec;99(6):1494-504. doi: 10.1111/j.1471-4159.2006.04176.x. J Neurochem. 2006. PMID: 17230639
-
[Serotonin receptor 1A-modulated dephosphorylation of glycine receptor α3: a new molecular mechanism of breathing control for compensation of opioid-induced respiratory depression without loss of analgesia].Schmerz. 2011 Jun;25(3):272-81. doi: 10.1007/s00482-011-1044-1. Schmerz. 2011. PMID: 21499860 Review. German.
-
Adenylyl cyclase--A-kinase anchoring protein complexes: the next dimension in cAMP signaling.Mol Pharmacol. 2009 Nov;76(5):935-41. doi: 10.1124/mol.109.059345. Epub 2009 Aug 14. Mol Pharmacol. 2009. PMID: 19684092 Free PMC article. Review.
Cited by
-
The delta opioid receptor tool box.Neuroscience. 2016 Dec 3;338:145-159. doi: 10.1016/j.neuroscience.2016.06.028. Epub 2016 Jun 24. Neuroscience. 2016. PMID: 27349452 Free PMC article. Review.
-
Revealing the Activity of Trimeric G-proteins in Live Cells with a Versatile Biosensor Design.Cell. 2020 Aug 6;182(3):770-785.e16. doi: 10.1016/j.cell.2020.06.020. Epub 2020 Jul 6. Cell. 2020. PMID: 32634377 Free PMC article.
-
Gα(i/o)-coupled receptor-mediated sensitization of adenylyl cyclase: 40 years later.Eur J Pharmacol. 2015 Sep 15;763(Pt B):223-32. doi: 10.1016/j.ejphar.2015.05.014. Epub 2015 May 14. Eur J Pharmacol. 2015. PMID: 25981304 Free PMC article. Review.
-
Blockade of endocannabinoid hydrolytic enzymes attenuates precipitated opioid withdrawal symptoms in mice.J Pharmacol Exp Ther. 2011 Oct;339(1):173-85. doi: 10.1124/jpet.111.181370. Epub 2011 Jun 30. J Pharmacol Exp Ther. 2011. PMID: 21719468 Free PMC article.
-
Dynorphin inhibits basal forebrain cholinergic neurons by pre- and postsynaptic mechanisms.J Physiol. 2016 Feb 15;594(4):1069-85. doi: 10.1113/JP271657. Epub 2016 Jan 5. J Physiol. 2016. PMID: 26613645 Free PMC article.
References
-
- Aghajanian GK. (1978) Tolerance of locus coeruleus neurones to morphine and suppression of withdrawal response by clonidine. Nature 276:186–188 - PubMed
-
- Allen JA, Halverson-Tamboli RA, Rasenick MM. (2007) Lipid raft microdomains and neurotransmitter signalling. Nat Rev Neurosci 8:128–140 - PubMed
-
- Alt A, McFadyen IJ, Fan CD, Woods JH, Traynor JR. (2001) Stimulation of guanosine-5′-O-(3-[35S]thio)triphosphate binding in digitonin-permeabilized C6 rat glioma cells: evidence for an organized association of mu-opioid receptors and G protein. J Pharmacol Exp Ther 298:116–121 - PubMed
-
- Aston-Jones G, Hirata H, Akaoka H. (1997) Local opiate withdrawal in locus coeruleus in vivo. Brain Res 765:331–336 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

