Immunization with a ZmpB-based protein vaccine could protect against pneumococcal diseases in mice
- PMID: 21098102
- PMCID: PMC3028838
- DOI: 10.1128/IAI.00717-10
Immunization with a ZmpB-based protein vaccine could protect against pneumococcal diseases in mice
Abstract
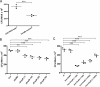
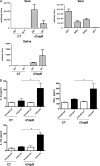
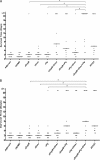
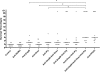
Zinc metalloprotease B (ZmpB) is present in all isolated pneumococcal strains and contributes to the pathogenesis of pneumococcal infection. In this study, recombinant ZmpB was cloned and expressed in Escherichia coli. The expression of ZmpB by different pneumococcal strains was detectable by Western blotting with antisera raised to recombinant ZmpB. Flow cytometry analysis demonstrated that anti-ZmpB polyclonal antibodies could bind to the cell surface of the pneumococcal strains analyzed. Both recombinant ZmpB protein and anti-ZmpB polyclonal antibodies significantly inhibited the adhesion of Streptococcus pneumoniae D39 to A549 cells. In mouse models, mucosal immunization with recombinant ZmpB could significantly reduce pneumococcal lung colonization caused by S. pneumoniae serotypes 19F and 14 and significantly increase mice survival times following invasive pneumococcal challenge with different pneumococcal strains, including serotypes 2, 3, 6B, and 14. Furthermore, intraperitoneal immunization with recombinant ZmpB in combination with the recombinant pneumolysin mutant (DeltaA146 Ply) and heat shock protein 40 (DnaJ) could enhance the protection against pneumococcal infection compared to protection provided by single-protein antigens. Passive immunization with hyperimmune antisera against these three antigens also demonstrated that the combination of three hyperimmune antisera could provide better protection than single antisera. Taken together, our results suggest that ZmpB is a good candidate pneumococcal vaccine antigen.
Figures




Similar articles
-
Immunization with a combination of three pneumococcal proteins confers additive and broad protection against Streptococcus pneumoniae Infections in Mice.Infect Immun. 2010 Mar;78(3):1276-83. doi: 10.1128/IAI.00473-09. Epub 2009 Dec 28. Infect Immun. 2010. PMID: 20038538 Free PMC article.
-
[Immunoprotective effect of combined pneumococcal endopeptidase O and pneumococcal surface adhesin A vaccines against Streptococcus pneumoniae infection].Zhongguo Dang Dai Er Ke Za Zhi. 2017 May;19(5):583-589. doi: 10.7499/j.issn.1008-8830.2017.05.021. Zhongguo Dang Dai Er Ke Za Zhi. 2017. PMID: 28506354 Free PMC article. Chinese.
-
Enhanced protection against pneumococcal infection elicited by immunization with the combination of PspA, PspC, and ClpP.Vaccine. 2007 Jun 28;25(27):4996-5005. doi: 10.1016/j.vaccine.2007.04.069. Epub 2007 May 11. Vaccine. 2007. PMID: 17524530
-
Streptococcus pneumoniae protein vaccine candidates: properties, activities and animal studies.Crit Rev Microbiol. 2006;32(3):139-53. doi: 10.1080/10408410600822942. Crit Rev Microbiol. 2006. PMID: 16893751 Review.
-
[Proteins of Streptococcus pneumoniae: perspectives for development of pneumococcal vaccine].Zh Mikrobiol Epidemiol Immunobiol. 2010 Nov-Dec;(6):98-104. Zh Mikrobiol Epidemiol Immunobiol. 2010. PMID: 21384595 Review. Russian.
Cited by
-
Pathogenicity and virulence of Streptococcus pneumoniae: Cutting to the chase on proteases.Virulence. 2021 Dec;12(1):766-787. doi: 10.1080/21505594.2021.1889812. Virulence. 2021. PMID: 33660565 Free PMC article. Review.
-
Mucosal immunization with recombinant fusion protein DnaJ-ΔA146Ply enhances cross-protective immunity against Streptococcus pneumoniae infection in mice via interleukin 17A.Infect Immun. 2014 Apr;82(4):1666-75. doi: 10.1128/IAI.01391-13. Epub 2014 Feb 3. Infect Immun. 2014. PMID: 24491576 Free PMC article.
-
An in vivo atlas of host-pathogen transcriptomes during Streptococcus pneumoniae colonization and disease.Proc Natl Acad Sci U S A. 2020 Dec 29;117(52):33507-33518. doi: 10.1073/pnas.2010428117. Epub 2020 Dec 14. Proc Natl Acad Sci U S A. 2020. PMID: 33318198 Free PMC article.
-
Serotype-independent protection against pneumococcal infections elicited by intranasal immunization with ethanol-killed pneumococcal strain, SPY1.J Microbiol. 2014 Apr;52(4):315-23. doi: 10.1007/s12275-014-3583-5. Epub 2014 Mar 29. J Microbiol. 2014. PMID: 24682994
-
The Role of Pneumococcal Virulence Factors in Ocular Infectious Diseases.Interdiscip Perspect Infect Dis. 2018 Nov 13;2018:2525173. doi: 10.1155/2018/2525173. eCollection 2018. Interdiscip Perspect Infect Dis. 2018. PMID: 30538741 Free PMC article. Review.
References
-
- Arvå, E., and B. Andersson. 1999. Induction of phagocyte-stimulating and Th1-promoting cytokines by in vitro stimulation of human peripheral blood mononuclear cells with Streptococcus pneumoniae. Scand. J. Immunol. 49:417-423. - PubMed
-
- Audouy, S. A., et al. 2007. Development of lactococcal GEM-based pneumococcal vaccines. Vaccine 25:2497-2506. - PubMed
-
- Beghetto, E., et al. 2006. Discovery of novel Streptococcus pneumoniae antigens by screening a whole-genome lambda λ-display library. FEMS. Microbiol. Lett. 262:14-21. - PubMed
-
- Bogaert, D., et al. 2004. Colonisation by Streptococcus pneumoniae and Staphylococcus aureus in healthy children. Lancet 363:1871-1872. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

