The Vi capsular polysaccharide prevents complement receptor 3-mediated clearance of Salmonella enterica serotype Typhi
- PMID: 21098104
- PMCID: PMC3028862
- DOI: 10.1128/IAI.00961-10
The Vi capsular polysaccharide prevents complement receptor 3-mediated clearance of Salmonella enterica serotype Typhi
Abstract
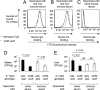
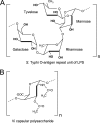
Capsular polysaccharides are important virulence factors of invasive bacterial pathogens. Here we studied the role of the virulence (Vi) capsular polysaccharide of Salmonella enterica serotype Typhi (S. Typhi) in preventing innate immune recognition by complement. Comparison of capsulated S. Typhi with a noncapsulated mutant (ΔtviBCDE vexABCDE mutant) revealed that the Vi capsule interfered with complement component 3 (C3) deposition. Decreased complement fixation resulted in reduced bacterial binding to complement receptor 3 (CR3) on the surface of murine macrophages in vitro and decreased CR3-dependent clearance of Vi capsulated S. Typhi from the livers and spleens of mice. Opsonization of bacteria with immune serum prior to intraperitoneal infection increased clearance of capsulated S. Typhi from the liver. Our data suggest that the Vi capsule prevents CR3-dependent clearance, which can be overcome in part by a specific antibody response.
Figures




References
-
- Clark, T. W., C. Daneshvar, M. Pareek, N. Perera, and I. Stephenson. 2010. Enteric fever in a UK regional infectious diseases unit: a 10 year retrospective review. J. Infect. 60:91-98. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI040124/AI/NIAID NIH HHS/United States
- C06 RR12088-01/RR/NCRR NIH HHS/United States
- T32 AI060555/AI/NIAID NIH HHS/United States
- R01 AI044170/AI/NIAID NIH HHS/United States
- R01 AI040124/AI/NIAID NIH HHS/United States
- AI088122/AI/NIAID NIH HHS/United States
- AI076246/AI/NIAID NIH HHS/United States
- R37 AI044170/AI/NIAID NIH HHS/United States
- AI044170/AI/NIAID NIH HHS/United States
- R21 AI073120/AI/NIAID NIH HHS/United States
- AI060555/AI/NIAID NIH HHS/United States
- C06 RR012088/RR/NCRR NIH HHS/United States
- R21 AI088122/AI/NIAID NIH HHS/United States
- R01 AI076246/AI/NIAID NIH HHS/United States
- AI073120/AI/NIAID NIH HHS/United States
- R29 AI040124/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

