Identification of gene products involved in the oxidative stress response of Moraxella catarrhalis
- PMID: 21098105
- PMCID: PMC3028835
- DOI: 10.1128/IAI.01060-10
Identification of gene products involved in the oxidative stress response of Moraxella catarrhalis
Abstract
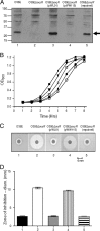
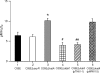
Moraxella catarrhalis is subjected to oxidative stress from both internal and environmental sources. A previous study (C. D. Pericone, K. Overweg, P. W. Hermans, and J. N. Weiser, Infect. Immun. 68:3990-3997, 2000) indicated that a wild-type strain of M. catarrhalis was very resistant to killing by exogenous hydrogen peroxide (H₂O₂). The gene encoding OxyR, a LysR family transcriptional regulator, was identified and inactivated in M. catarrhalis strain O35E, resulting in an increase in sensitivity to killing by H₂O₂ in disk diffusion assays and a concomitant aerobic serial dilution effect. Genes encoding a predicted catalase (KatA) and an alkyl hydroperoxidase (AhpCF) showed dose-dependent upregulation in wild-type cells exposed to H₂O₂. DNA microarray and real-time reverse transcription-PCR (RT-PCR) analyses identified M. catarrhalis genes whose expression was affected by oxidative stress in an OxyR-dependent manner. Testing of M. catarrhalis O35E katA and ahpC mutants for their abilities to scavenge exogenous H₂O₂ showed that the KatA catalase was responsible for most of this activity in the wild-type parent strain. The introduction of the same mutations into M. catarrhalis strain ETSU-4 showed that the growth of a ETSU-4 katA mutant was markedly inhibited by the addition of 50 mM H₂O₂ but that this mutant could still form a biofilm equivalent to that produced by its wild-type parent strain.
Figures



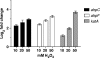
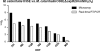
Similar articles
-
A Moraxella catarrhalis two-component signal transduction system necessary for growth in liquid media affects production of two lysozyme inhibitors.Infect Immun. 2015 Jan;83(1):146-60. doi: 10.1128/IAI.02486-14. Epub 2014 Oct 13. Infect Immun. 2015. PMID: 25312959 Free PMC article.
-
OxyR mediated compensatory expression between ahpC and katA and the significance of ahpC in protection from hydrogen peroxide in Xanthomonas campestris.FEMS Microbiol Lett. 2005 Aug 1;249(1):73-8. doi: 10.1016/j.femsle.2005.06.002. FEMS Microbiol Lett. 2005. PMID: 15993009
-
Identification of a repressor of a truncated denitrification pathway in Moraxella catarrhalis.J Bacteriol. 2008 Dec;190(23):7762-72. doi: 10.1128/JB.01032-08. Epub 2008 Sep 26. J Bacteriol. 2008. PMID: 18820017 Free PMC article.
-
A hag mutant of Moraxella catarrhalis strain O35E is deficient in hemagglutination, autoagglutination, and immunoglobulin D-binding activities.Infect Immun. 2002 Aug;70(8):4523-33. doi: 10.1128/IAI.70.8.4523-4533.2002. Infect Immun. 2002. PMID: 12117964 Free PMC article.
-
Inactivation of the Moraxella catarrhalis superoxide dismutase SodA induces constitutive expression of iron-repressible outer membrane proteins.Infect Immun. 2002 Apr;70(4):1889-95. doi: 10.1128/IAI.70.4.1889-1895.2002. Infect Immun. 2002. PMID: 11895952 Free PMC article.
Cited by
-
The Moraxella catarrhalis phase-variable DNA methyltransferase ModM3 is an epigenetic regulator that affects bacterial survival in an in vivo model of otitis media.BMC Microbiol. 2019 Dec 9;19(1):276. doi: 10.1186/s12866-019-1660-y. BMC Microbiol. 2019. PMID: 31818247 Free PMC article.
-
Influence of oxyR on Growth, Biofilm Formation, and Mobility of Vibrio parahaemolyticus.Appl Environ Microbiol. 2015 Nov 20;82(3):788-96. doi: 10.1128/AEM.02818-15. Print 2016 Feb 1. Appl Environ Microbiol. 2015. PMID: 26590276 Free PMC article.
-
Acinetobacter baumannii OxyR Regulates the Transcriptional Response to Hydrogen Peroxide.Infect Immun. 2018 Dec 19;87(1):e00413-18. doi: 10.1128/IAI.00413-18. Print 2019 Jan. Infect Immun. 2018. PMID: 30297527 Free PMC article.
-
A Moraxella catarrhalis two-component signal transduction system necessary for growth in liquid media affects production of two lysozyme inhibitors.Infect Immun. 2015 Jan;83(1):146-60. doi: 10.1128/IAI.02486-14. Epub 2014 Oct 13. Infect Immun. 2015. PMID: 25312959 Free PMC article.
-
Expression of the Oligopeptide Permease Operon of Moraxella catarrhalis Is Regulated by Temperature and Nutrient Availability.Infect Immun. 2015 Sep;83(9):3497-505. doi: 10.1128/IAI.00597-15. Epub 2015 Jun 22. Infect Immun. 2015. PMID: 26099587 Free PMC article.
References
-
- Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. - PubMed
-
- Borrill, Z. L., K. Roy, and D. Singh. 2008. Exhaled breath condensate biomarkers in COPD. Eur. Respir. J. 32:472-486. - PubMed
-
- Budhani, R. K., and J. K. Struthers. 1998. Interaction of Streptococcus pneumoniae and Moraxella catarrhalis: investigation of the indirect pathogenic role of beta-lactamase-producing moraxellae by use of a continuous-culture biofilm system. Antimicrob. Agents Chemother. 42:2521-2526. - PMC - PubMed
-
- Choi, H., et al. 2001. Structural basis of the redox switch in the OxyR transcription factor. Cell 105:103-113. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

