Receptor interacting protein kinases mediate retinal detachment-induced photoreceptor necrosis and compensate for inhibition of apoptosis
- PMID: 21098270
- PMCID: PMC3003048
- DOI: 10.1073/pnas.1009179107
Receptor interacting protein kinases mediate retinal detachment-induced photoreceptor necrosis and compensate for inhibition of apoptosis
Abstract
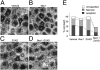
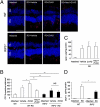
Apoptosis has been shown to be a significant form of cell loss in many diseases. Detachment of photoreceptors from the retinal pigment epithelium, as seen in various retinal disorders, causes photoreceptor loss and subsequent vision decline. Although caspase-dependent apoptotic pathways are activated after retinal detachment, caspase inhibition by the pan-caspase inhibitor Z-VAD fails to prevent photoreceptor death; thus, we investigated other pathways leading to cell loss. Here, we show that receptor interacting protein (RIP) kinase-mediated necrosis is a significant mode of photoreceptor cell loss in an experimental model of retinal detachment and when caspases are inhibited, RIP-mediated necrosis becomes the predominant form of death. RIP3 expression, a key activator of RIP1 kinase, increased more than 10-fold after retinal detachment. Morphological assessment of detached retinas treated with Z-VAD showed decreased apoptosis but significantly increased necrotic photoreceptor death. RIP1 kinase inhibitor necrostatin-1 or Rip3 deficiency substantially prevented those necrotic changes and reduced oxidative stress and mitochondrial release of apoptosis-inducing factor. Thus, RIP kinase-mediated programmed necrosis is a redundant mechanism of photoreceptor death in addition to apoptosis, and simultaneous inhibition of RIP kinases and caspases is essential for effective neuroprotection and may be a novel therapeutic strategy for treatment of retinal disorders.
Conflict of interest statement
Conflict of interest statement: G.T. and D.G.V. have a provisional patent filling.
Figures






References
-
- Dunaief JL, Dentchev T, Ying GS, Milam AH. The role of apoptosis in age-related macular degeneration. Arch Ophthalmol. 2002;120:1435–1442. - PubMed
-
- Cook B, Lewis GP, Fisher SK, Adler R. Apoptotic photoreceptor degeneration in experimental retinal detachment. Invest Ophthalmol Vis Sci. 1995;36:990–996. - PubMed
-
- Arroyo JG, Yang L, Bula D, Chen DF. Photoreceptor apoptosis in human retinal detachment. Am J Ophthalmol. 2005;139:605–610. - PubMed
-
- Campo RV, et al. Pars plana vitrectomy without scleral buckle for pseudophakic retinal detachments. Ophthalmology. 1999;106:1811–1815. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

