Trial-to-trial variability of the prefrontal neurons reveals the nature of their engagement in a motion discrimination task
- PMID: 21098286
- PMCID: PMC3003075
- DOI: 10.1073/pnas.1009956107
Trial-to-trial variability of the prefrontal neurons reveals the nature of their engagement in a motion discrimination task
Abstract
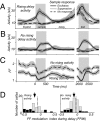
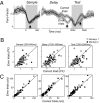
During motion discrimination tasks, many prefrontal cortex (PFC) neurons are strongly modulated by the behavioral context, suggesting their involvement in sensory discriminations. Recent studies suggest that trial-to-trial variability of spiking activity characteristic of cortical neurons could be a source of information about the state of neurons and their participation in behavioral tasks. We tested this hypothesis by examining the variability of putative pyramidal PFC neurons, a likely source of top-down influences. The variability of these neurons was calculated as a ratio of spike count variance to its mean (fano factor, FF), while monkeys compared the directions of two moving stimuli, sample and test, separated by a delay. We found that the FF tracked consecutive components of the task, dropping rapidly with the onset of stimuli being discriminated and declining more slowly before each salient event of the trial: The sample, the test, and the response. These time-dependent signals were less consistent in direction selective neurons and were largely absent during passive fixation. Furthermore, neurons with test responses that reflected the remembered sample decreased their FF well before the test, revealing the predictive nature of response variability, an effect present only during the active task. The FF was also sensitive to behavioral performance, exhibiting different temporal dynamics on error trials. These changes did not depend on firing rates and were often the only metric correlated with task demands. Our results demonstrate that trial-to-trial variability provides a sensitive measure of the engagement of putative pyramidal PFC neurons in circuits subserving discrimination tasks.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


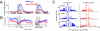


References
-
- Miller EK. The prefrontal cortex and cognitive control. Nat Rev Neurosci. 2000;1:59–65. - PubMed
-
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. - PubMed
-
- Freedman DJ, Riesenhuber M, Poggio T, Miller EK. Categorical representation of visual stimuli in the primate prefrontal cortex. Science. 2001;291:312–316. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

