Biguanide metformin acts on tau phosphorylation via mTOR/protein phosphatase 2A (PP2A) signaling
- PMID: 21098287
- PMCID: PMC3003072
- DOI: 10.1073/pnas.0912793107
Biguanide metformin acts on tau phosphorylation via mTOR/protein phosphatase 2A (PP2A) signaling
Abstract
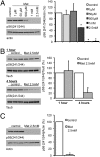
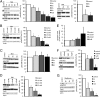
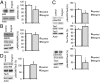
Hyperphosphorylated tau plays an important role in the formation of neurofibrillary tangles in brains of patients with Alzheimer's disease (AD) and related tauopathies and is a crucial factor in the pathogenesis of these disorders. Though diverse kinases have been implicated in tau phosphorylation, protein phosphatase 2A (PP2A) seems to be the major tau phosphatase. Using murine primary neurons from wild-type and human tau transgenic mice, we show that the antidiabetic drug metformin induces PP2A activity and reduces tau phosphorylation at PP2A-dependent epitopes in vitro and in vivo. This tau dephosphorylating potency can be blocked entirely by the PP2A inhibitors okadaic acid and fostriecin, confirming that PP2A is an important mediator of the observed effects. Surprisingly, metformin effects on PP2A activity and tau phosphorylation seem to be independent of AMPK activation, because in our experiments (i) metformin induces PP2A activity before and at lower levels than AMPK activity and (ii) the AMPK activator AICAR does not influence the phosphorylation of tau at the sites analyzed. Affinity chromatography and immunoprecipitation experiments together with PP2A activity assays indicate that metformin interferes with the association of the catalytic subunit of PP2A (PP2Ac) to the so-called MID1-α4 protein complex, which regulates the degradation of PP2Ac and thereby influences PP2A activity. In summary, our data suggest a potential beneficial role of biguanides such as metformin in the prophylaxis and/or therapy of AD.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


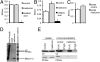
References
-
- Gong CX, Liu F, Grundke-Iqbal I, Iqbal K. Post-translational modifications of tau protein in Alzheimer's disease. J Neural Transm. 2005;112:813–838. - PubMed
-
- Alonso AD, Grundke-Iqbal I, Barra HS, Iqbal K. Abnormal phosphorylation of tau and the mechanism of Alzheimer neurofibrillary degeneration: Sequestration of microtubule-associated proteins 1 and 2 and the disassembly of microtubules by the abnormal tau. Proc Natl Acad Sci USA. 1997;94:298–303. - PMC - PubMed
-
- Hanger DP, Betts JC, Loviny TL, Blackstock WP, Anderton BH. New phosphorylation sites identified in hyperphosphorylated tau (paired helical filament-tau) from Alzheimer's disease brain using nanoelectrospray mass spectrometry. J Neurochem. 1998;71:2465–2476. - PubMed
-
- Goedert M, Jakes R, Qi Z, Wang JH, Cohen P. Protein phosphatase 2A is the major enzyme in brain that dephosphorylates tau protein phosphorylated by proline-directed protein kinases or cyclic AMP-dependent protein kinase. J Neurochem. 1995;65:2804–2807. - PubMed
-
- Sontag E, Nunbhakdi-Craig V, Lee G, Bloom GS, Mumby MC. Regulation of the phosphorylation state and microtubule-binding activity of Tau by protein phosphatase 2A. Neuron. 1996;17:1201–1207. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

