Strategies for stabilizing superoxide dismutase (SOD1), the protein destabilized in the most common form of familial amyotrophic lateral sclerosis
- PMID: 21098299
- PMCID: PMC3003092
- DOI: 10.1073/pnas.1015463107
Strategies for stabilizing superoxide dismutase (SOD1), the protein destabilized in the most common form of familial amyotrophic lateral sclerosis
Abstract
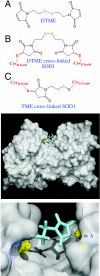
Amyotrophic lateral sclerosis (ALS) is a disorder characterized by the death of both upper and lower motor neurons and by 3- to 5-yr median survival postdiagnosis. The only US Food and Drug Administration-approved drug for the treatment of ALS, Riluzole, has at best, moderate effect on patient survival and quality of life; therefore innovative approaches are needed to combat neurodegenerative disease. Some familial forms of ALS (fALS) have been linked to mutations in the Cu/Zn superoxide dismutase (SOD1). The dominant inheritance of mutant SOD1 and lack of symptoms in knockout mice suggest a "gain of toxic function" as opposed to a loss of function. A prevailing hypothesis for the mechanism of the toxicity of fALS-SOD1 variants, or the gain of toxic function, involves dimer destabilization and dissociation as an early step in SOD1 aggregation. Therefore, stabilizing the SOD1 dimer, thus preventing aggregation, is a potential therapeutic strategy. Here, we report a strategy in which we chemically cross-link the SOD1 dimer using two adjacent cysteine residues on each respective monomer (Cys111). Stabilization, measured as an increase in melting temperature, of ∼20 °C and ∼45 °C was observed for two mutants, G93A and G85R, respectively. This stabilization is the largest for SOD1, and to the best of our knowledge, for any disease-related protein. In addition, chemical cross-linking conferred activity upon G85R, an otherwise inactive mutant. These results demonstrate that targeting these cysteine residues is an important new strategy for development of ALS therapies.
Conflict of interest statement
Conflict of interest statement: A patent has been filed by the authors relating to the strategy of SOD1 stabilization described here.
Figures



References
-
- Bensimon G, Lacomblez L, Meininger V. A controlled trial of riluzole in amyotrophic lateral sclerosis ALS/Riluzole Study Group. N Engl J Med. 1994;330:585–591. - PubMed
-
- Lacomblez L, Bensimon G, Leigh PN, Guillet P, Meininger V. Dose-ranging study of riluzole in amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis/Riluzole Study Group II. Lancet. 1996;347:1425–1431. - PubMed
-
- Wagner ML, Landis BE. Riluzole: A new agent for amyotrophic lateral sclerosis. Ann Pharmacother. 1997;31:738–744. - PubMed
-
- Deng HX, et al. Amyotrophic lateral sclerosis and structural defects in Cu, Zn superoxide dismutase. Science. 1993;261:1047–1051. - PubMed
-
- Rosen DR, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

