Immunoglobulin light chains activate nuclear factor-κB in renal epithelial cells through a Src-dependent mechanism
- PMID: 21098396
- PMCID: PMC3056473
- DOI: 10.1182/blood-2010-08-302505
Immunoglobulin light chains activate nuclear factor-κB in renal epithelial cells through a Src-dependent mechanism
Abstract
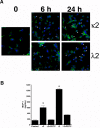
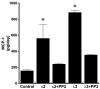
One of the major attendant complications of multiple myeloma is renal injury, which contributes significantly to morbidity and mortality in this disease. Monoclonal immunoglobulin free light chains (FLCs) are usually directly involved, and tubulointerstitial renal injury and fibrosis are prominent histologic features observed in myeloma. The present study examined the role of monoclonal FLCs in altering the nuclear factor κ light chain enhancer of activated B cells (NF-κB) activity of renal epithelial cells. Human proximal tubule epithelial cells exposed to 3 different human monoclonal FLCs demonstrated Src kinase-dependent activation of the NF-κB pathway, which increased production of monocyte chemoattractant protein-1 (MCP-1). Tyrosine phosphorylation of inhibitor of κB kinases (IKKs) IKKα and IKKβ and a concomitant increase in inhibitor of κB (IκB) kinase activity in cell lysates were observed. Time-dependent, Src kinase-dependent increases in serine and tyrosine phosphorylation of IκBα and NF-κB activity were also demonstrated. Proteasome inhibition partially blocked FLC-induced MCP-1 production. These findings fit into a paradigm characterized by FLC-induced redox-signaling events that activated the canonical and atypical (IKK-independent) NF-κB pathways to promote a proinflammatory, profibrotic renal environment.
Figures





References
-
- Greenlee RT, Murray T, Bolden S, Wingo PA. Cancer statistics, 2000. CA Cancer J Clin. 2000;50(1):7–33. - PubMed
-
- Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78(1):21–33. - PubMed
-
- Abbott KC, Agodoa LY. Multiple myeloma and light chain-associated nephropathy at end-stage renal disease in the United States: patient characteristics and survival. Clin Nephrol. 2001;56(3):207–210. - PubMed
-
- Sanders PW. Myeloma and secondary involvement of the kidney in dysproteinemias. In: Wilcox CS, editor. Therapy in nephrology and hypertension: a companion to Brenner and Rector's the kidney. 5th ed. London, United Kingdom: WB Saunders; 2008. pp. 461–468.
-
- Batuman V, Dreisbach AW, Cyran J. Light-chain binding sites on renal brush-border membranes. Am J Physiol. 1990;258(5 Pt 2):F1259–F1265. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

