Caveolin-1 assembles type 1 inositol 1,4,5-trisphosphate receptors and canonical transient receptor potential 3 channels into a functional signaling complex in arterial smooth muscle cells
- PMID: 21098487
- PMCID: PMC3039319
- DOI: 10.1074/jbc.M110.179747
Caveolin-1 assembles type 1 inositol 1,4,5-trisphosphate receptors and canonical transient receptor potential 3 channels into a functional signaling complex in arterial smooth muscle cells
Abstract
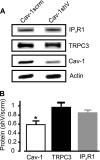
Physical coupling of sarcoplasmic reticulum (SR) type 1 inositol 1,4,5-trisphosphate receptors (IP(3)R1) to plasma membrane canonical transient receptor potential 3 (TRPC3) channels activates a cation current (I(Cat)) in arterial smooth muscle cells that induces vasoconstriction. However, structural components that enable IP(3)R1 and TRPC3 channels to communicate locally are unclear. Caveolae are plasma membrane microdomains that can compartmentalize proteins. Here, we tested the hypothesis that caveolae and specifically caveolin-1 (cav-1), a caveolae scaffolding protein, facilitate functional IP(3)R1 to TRPC3 coupling in smooth muscle cells of resistance-size cerebral arteries. Methyl-β-cyclodextrin (MβCD), which disassembles caveolae, reduced IP(3)-induced I(Cat) activation in smooth muscle cells and vasoconstriction in pressurized arteries. Cholesterol replenishment reversed these effects. Cav-1 knockdown using shRNA attenuated IP(3)-induced vasoconstriction, but did not alter TRPC3 and IP(3)R1 expression. A synthetic peptide corresponding to the cav-1 scaffolding domain (CSD) sequence (amino acids 82-101) also attenuated IP(3)-induced I(Cat) activation and vasoconstriction. A cav-1 antibody co-immunoprecipitated cav-1, TRPC3, and IP(3)R1 from cerebral artery lysate. ImmunoFRET indicated that cav-1, TRPC3 channels and IP(3)R1 are spatially co-localized in arterial smooth muscle cells. IP(3)R1 and TRPC3 channel spatial localization was disrupted by MβCD and a CSD peptide. Cholesterol replenishment re-established IP(3)R1 and TRPC3 channel close spatial proximity. Taken together, these data indicate that in arterial smooth muscle cells, cav-1 co-localizes SR IP(3)R1 and plasma membrane TRPC3 channels in close spatial proximity thereby enabling IP(3)-induced physical coupling of these proteins, leading to I(Cat) generation and vasoconstriction.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

