Efavirenz concentrations in CSF exceed IC50 for wild-type HIV
- PMID: 21098541
- PMCID: PMC3019085
- DOI: 10.1093/jac/dkq434
Efavirenz concentrations in CSF exceed IC50 for wild-type HIV
Abstract
Objectives: HIV-associated neurocognitive disorders remain common despite use of potent antiretroviral therapy (ART). Ongoing viral replication due to poor distribution of antivirals into the CNS may increase risk for HIV-associated neurocognitive disorders. This study's objective was to determine penetration of a commonly prescribed antiretroviral drug, efavirenz, into CSF.
Methods: CHARTER is an ongoing, North American, multicentre, observational study to determine the effects of ART on HIV-associated neurological disease. Single random plasma and CSF samples were drawn within 1 h of each other from subjects taking efavirenz between September 2003 and July 2007. Samples were assayed by HPLC or HPLC/mass spectrometry with detection limits of 39 ng/mL (plasma) and <0.1 ng/mL (CSF).
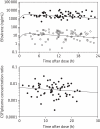
Results: Eighty participants (age 44 ± 8 years; 79 ± 15 kg; 20 females) had samples drawn 12.5 ± 5.4 h post-dose. The median efavirenz concentrations after a median of 7 months [interquartile range (IQR) 2-17] of therapy were 2145 ng/mL in plasma (IQR 1384-4423) and 13.9 ng/mL in CSF (IQR 4.1-21.2). The CSF/plasma concentration ratio from paired samples drawn within 1 h of each other was 0.005 (IQR 0.0026-0.0076; n = 69). The CSF/IC(50) ratio was 26 (IQR 8-41) using the published IC(50) for wild-type HIV (0.51 ng/mL). Two CSF samples had concentrations below the efavirenz IC(50) for wild-type HIV.
Conclusions: Efavirenz concentrations in the CSF are only 0.5% of plasma concentrations but exceed the wild-type IC(50) in nearly all individuals. Since CSF drug concentrations reflect those in brain interstitial fluids, efavirenz reaches therapeutic concentrations in brain tissue.
Figures
References
-
- Simioni S, Cavassini M, Annoni JM, et al. Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS. 2010;24:1243–50. - PubMed
-
- Reddy YS, Kashuba A, Gerber J, et al. Roundtable report: importance of antiretroviral drug concentrations in sanctuary sites and viral reservoirs. AIDS Res Hum Retroviruses. 2003;19:167–76. doi:10.1089/088922203763315669. - DOI - PubMed
-
- van Praag RM, van Weert EC, van Heeswijk RP, et al. Stable concentrations of zidovudine, stavudine, lamivudine, abacavir, and nevirapine in serum and cerebrospinal fluid during 2 years of therapy. Antimicrob Agents Chemother. 2002;46:896–9. doi:10.1128/AAC.46.3.896-899.2002. - DOI - PMC - PubMed
-
- Tashima KT, Caliendo AM, Ahmad M, et al. Cerebrospinal fluid human immunodeficiency virus type 1 (HIV-1) suppression and efavirenz drug concentrations in HIV-1-infected patients receiving combination therapy. J Infect Dis. 1999;180:862–4. doi:10.1086/314945. - DOI - PubMed
-
- Antinori A, Perno CF, Giancola ML, et al. Efficacy of cerebrospinal fluid (CSF)-penetrating antiretroviral drugs against HIV in the neurological compartment: different patterns of phenotypic resistance in CSF and plasma. Clin Infect Dis. 2005;41:1787–93. doi:10.1086/498310. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

