Initiation of trophectoderm lineage specification in mouse embryos is independent of Cdx2
- PMID: 21098565
- PMCID: PMC2990207
- DOI: 10.1242/dev.056630
Initiation of trophectoderm lineage specification in mouse embryos is independent of Cdx2
Abstract
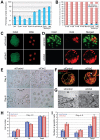
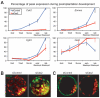
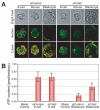
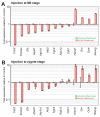
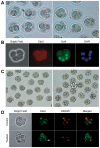
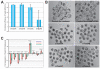
The separation of the first two lineages - trophectoderm (TE) and inner cell mass (ICM) - is a crucial event in the development of the early embryo. The ICM, which constitutes the pluripotent founder cell population, develops into the embryo proper, whereas the TE, which comprises the surrounding outer layer, supports the development of the ICM before and after implantation. Cdx2, the first transcription factor expressed specifically in the developing TE, is crucial for the differentiation of cells into the TE, as lack of zygotic Cdx2 expression leads to a failure of embryos to hatch and implant into the uterus. However, speculation exists as to whether maternal Cdx2 is required for initiation of TE lineage separation. Here, we show that effective elimination of both maternal and zygotic Cdx2 transcripts by an RNA interference approach resulted in failure of embryo hatching and implantation, but the developing blastocysts exhibited normal gross morphology, indicating that TE differentiation had been initiated. Expression of keratin 8, a marker for differentiated TE, further confirmed the identity of the TE lineage in Cdx2-deficient embryos. However, these embryos exhibited low mitochondrial activity and abnormal ultrastructure, indicating that Cdx2 plays a key role in the regulation of TE function. Furthermore, we found that embryonic compaction does not act as a 'switch' regulator to turn on Cdx2 expression. Our results clearly demonstrate that neither maternal nor zygotic Cdx2 transcripts direct the initiation of ICM/TE lineage separation.
Figures
References
-
- Bai Y. Q., Yamamoto H., Akiyama Y., Tanaka H., Takizawa T., Koike M., Kenji Yagi O., Saitoh K., Takeshita K., Iwai T., et al. (2002). Ectopic expression of homeodomain protein CDX2 in intestinal metaplasia and carcinomas of the stomach. Cancer Lett. 176, 47-55 - PubMed
-
- Boiani M., Gambles V., Schöler H. R. (2004). ATP levels in clone mouse embryos. Cytogenet. Genome Res. 105, 270-278
-
- Boiani M., Gentile L., Gambles V. V., Cavaleri F., Redi C. A., Scholer H. R. (2005). Variable reprogramming of the pluripotent stem cell marker Oct4 in mouse clones: distinct developmental potentials in different culture environments. Stem Cells 23, 1089-1104 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

