Thrombolysis in very elderly people: controlled comparison of SITS International Stroke Thrombolysis Registry and Virtual International Stroke Trials Archive
- PMID: 21098614
- PMCID: PMC2990864
- DOI: 10.1136/bmj.c6046
Thrombolysis in very elderly people: controlled comparison of SITS International Stroke Thrombolysis Registry and Virtual International Stroke Trials Archive
Abstract
Objective: To assess effect of age on response to alteplase in acute ischaemic stroke.
Design: Adjusted controlled comparison of outcomes between non-randomised patients who did or did not undergo thrombolysis. Analysis used Cochran-Mantel-Haenszel test and proportional odds logistic regression analysis.
Setting: Collaboration between International Stroke Thrombolysis Registry (SITS-ISTR) and Virtual International Stroke Trials Archive (VISTA).
Participants: 23 334 patients from SITS-ISTR (December 2002 to November 2009) who underwent thrombolysis and 6166 from VISTA neuroprotection trials (1998-2007) who did not undergo thrombolysis (as controls). Of the 29 500 patients (3472 aged >80 ("elderly," mean 84.6), data on 272 patients were missing for baseline National Institutes of Health stroke severity score, leaving 29 228 patients for analysis adjusted for age and baseline severity.
Main outcome measures: Functional outcomes at 90 days measured by score on modified Rankin scale.
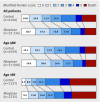
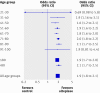
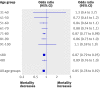
Results: Median severity at baseline was the same for patients who underwent thrombolysis and controls (median baseline stroke scale score: 12 for each group, P=0.14; n=29 228). The distribution of scores on the modified Rankin scale was better among all thrombolysis patients than controls (odds ratio 1.6, 95% confidence interval 1.5 to 1.7; Cochran-Mantel-Haenszel P<0.001). The association occurred independently among patients aged ≤80 (1.6, 1.5 to 1.7; P<0.001; n=25 789) and in those aged >80 (1.4, 1.3 to 1.6; P<0.001; n=3439). Odds ratios were consistent across all 10 year age ranges above 30, and benefit was significant from age 41 to 90; dichotomised outcomes (score on modified Rankin scale 0-1 v 2-6; 0-2 v 3-6; and 6 (death) v rest) were consistent with the results of the ordinal analysis.
Conclusions: Outcome in patients with acute ischaemic stroke is significantly better in those who undergo thrombolysis compared with those who do not. Increasing age is associated with poorer outcome but the association between thrombolysis treatment and improved outcome is maintained in very elderly people. Age alone should not be a barrier to treatment.
Conflict of interest statement
Funding: VISTA has received financial support from the European Stroke Organisation in the form of an unrestricted grant and contributions towards data extraction and capacity building from the Universities of Glasgow, California San Diego, Nottingham, Edmonton, Calgary, Texas, and Massachusetts; from commercial groups including Brainsgate, Novartis, Boehringer Ingelheim, and the Vertical Group; and from grant agencies and charities including the UK Stroke Association. SITS-ISTR is funded by an unrestricted grant from Boehringer Ingelheim, Ferrer, and a grant from European Union Public Health Executive Authority (PHEA). Financial support was also provided through the regional agreement on medical training and research (ALF) between Stockholm County Council and the Karolinska Institute. NKM is supported by a Scottish Overseas Research Studentship, a University of Glasgow scholarship, and an educational grant from the European Stroke Organisation for Young Neurologists. PJL is supported by the Finnish Academy and Sigrid Juselius foundation and Helsinki University Hospital research funds (EVO).
Competing interests: All authors have completed the Unified Competing Interest form at
Figures


Comment in
-
Intravenous thrombolysis for stroke.BMJ. 2010 Nov 23;341:c5891. doi: 10.1136/bmj.c5891. BMJ. 2010. PMID: 21098612 No abstract available.
-
Thrombolysis in elderly people. Observational data insufficient to change treatment.BMJ. 2011 Jan 19;342:d306; author reply d312. doi: 10.1136/bmj.d306. BMJ. 2011. PMID: 21248009 No abstract available.
-
Thrombolysis improves 90-day functional outcome in acute ischaemic stroke, even in very older people.Evid Based Med. 2011 Oct;16(5):136-7. doi: 10.1136/ebm1302. Epub 2011 Mar 29. Evid Based Med. 2011. PMID: 21447524 No abstract available.
-
Alter allein ist keine Kontraindikation für Thrombolyse bei ischämischem Schlaganfall.Praxis (Bern 1994). 2011 Jul 6;100(14):872-3. doi: 10.1024/1661-8157/a000597. Praxis (Bern 1994). 2011. PMID: 21732303 German. No abstract available.
References
-
- Generalized efficacy of t-PA for acute stroke. Subgroup analysis of the NINDS t-PA Stroke Trial. Stroke 1997;28:2119-25. - PubMed
-
- National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995;333:1581-7. - PubMed
-
- Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008;359:1317-29. - PubMed
-
- Lees KR, Bluhmki E, von Kummer R, Brott TG, Toni D, Grotta JC, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet 2010;375:1695-703. - PubMed
-
- Ahmed N, Wahlgren N, Grond M, Hennerici M, Lees KR, Mikulik R, et al. Implementation and outcome of thrombolysis with alteplase 3-4.5 h after an acute stroke: an updated analysis from SITS-ISTR. Lancet Neurol 2010;9:866-74. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
