p21CIP-1/WAF-1 induction is required to inhibit prostate cancer growth elicited by deficient expression of the Wnt inhibitor Dickkopf-1
- PMID: 21098705
- PMCID: PMC3059079
- DOI: 10.1158/0008-5472.CAN-10-0440
p21CIP-1/WAF-1 induction is required to inhibit prostate cancer growth elicited by deficient expression of the Wnt inhibitor Dickkopf-1
Abstract
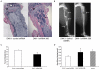
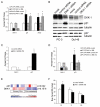
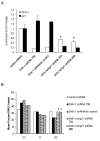
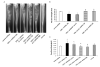
Osteoblastic bone metastases are the most common metastases produced by human prostate cancers (PCa). Deregulated activity of Wnt growth factors resulting from overexpression of the Wnt inhibitor Dickkopf-1 (DKK-1) is known to contribute to formation of the osteoblastic component of PCa skeletal bone metastases. In this study, we report that DKK-1 knockdown in osteolytic human PCa cells unexpectedly delays the development of both soft tissue and osseous lesions. PCa cells deficient in DKK-1 expression did not increase canonical Wnt signaling in target osteoblast cell lines; however, DKK-1 knockdown PCa cells exhibited increased expression of the CDK inhibitor p21(CIP1/WAF1) and a 32% increase in G(1) arrest compared with control cells. Ablating p21(CIP1/WAF1) in PCa cells deficient in DKK-1 was sufficient to rescue tumor growth. Collectively, our findings demonstrate that DKK-1 overexpression supports tumor growth in part by restricting expression of p21(CIP1/WAF1) through a mechanism independent of canonical Wnt signaling.
Figures

References
-
- Keller ET, Zhang J, Cooper CR, et al. Prostate carcinoma skeletal metastases: cross-talk between tumor and bone. Cancer Metastasis Rev. 2001;20:333–49. - PubMed
-
- Bubendorf L, Schopfer A, Wagner U, et al. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol. 2000;31:578–83. - PubMed
-
- Roudier MP, Corey E, True LD, et al. Histological, immunophenotypic and histomorphometric characterization of prostate cancer bone metastases. Cancer Treat Res. 2004;118:311–39. - PubMed
-
- Logothetis CJ, Lin SH. Osteoblasts in prostate cancer metastasis to bone. Nat Rev Cancer. 2005;5:21–8. - PubMed
-
- Hall CL, Bafico A, Dai J, Aaronson SA, Keller ET. Prostate cancer cells promote osteoblastic bone metastases through Wnts. Cancer Res. 2005;65:7554–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

