LFA-1-specific therapy prolongs allograft survival in rhesus macaques
- PMID: 21099108
- PMCID: PMC2994340
- DOI: 10.1172/JCI43895
LFA-1-specific therapy prolongs allograft survival in rhesus macaques
Abstract
Outcomes in transplantation have been limited by suboptimal long-term graft survival and toxicities associated with current immunosuppressive approaches. T cell costimulation blockade has shown promise as an alternative strategy to avoid the side effects of conventional immunosuppressive therapies, but targeting CD28-mediated costimulation alone has proven insufficient to prevent graft rejection in primates. Donor-specific memory T (TM) cells have been implicated in costimulation blockade-resistant transplant rejection, due to their enhanced effector function and decreased reliance on costimulatory signaling. Thus, we have tested a potential strategy to overcome TM cell-driven rejection by targeting molecules preferentially expressed on these cells, such as the adhesion molecule lymphocyte function-associated antigen 1 (LFA-1). Here, we show that short-term treatment (i.e., induction therapy) with the LFA-1-specific antibody TS-1/22 in combination with either basiliximab (an IL-2Rα-specific mAb) and sirolimus (a mammalian target of rapamycin inhibitor) or belatacept (a high-affinity variant of the CD28 costimulation-blocker CTLA4Ig) prolonged islet allograft survival in nonhuman primates relative to control treatments. Moreover, TS-1/22 masked LFA-1 on TM cells in vivo and inhibited the generation of alloproliferative and cytokine-producing effector T cells that expressed high levels of LFA-1 in vitro. These results support the use of LFA-1-specific induction therapy to neutralize costimulation blockade-resistant populations of T cells and further evaluation of LFA-1-specific therapeutics for use in transplantation.
Figures




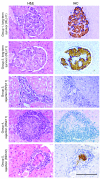

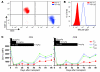
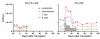
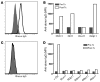
References
-
- Lafferty KJ, Cunningham AJ. A new analysis of allogeneic interactions. Aust J Exp Biol Med Sci. 1975;53(1):27–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

