Hepcidin as a therapeutic tool to limit iron overload and improve anemia in β-thalassemic mice
- PMID: 21099112
- PMCID: PMC2993583
- DOI: 10.1172/JCI41717
Hepcidin as a therapeutic tool to limit iron overload and improve anemia in β-thalassemic mice
Abstract
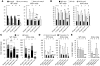
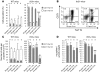
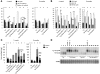
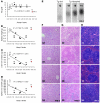
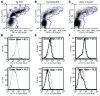
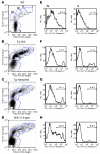
Excessive iron absorption is one of the main features of β-thalassemia and can lead to severe morbidity and mortality. Serial analyses of β-thalassemic mice indicate that while hemoglobin levels decrease over time, the concentration of iron in the liver, spleen, and kidneys markedly increases. Iron overload is associated with low levels of hepcidin, a peptide that regulates iron metabolism by triggering degradation of ferroportin, an iron-transport protein localized on absorptive enterocytes as well as hepatocytes and macrophages. Patients with β-thalassemia also have low hepcidin levels. These observations led us to hypothesize that more iron is absorbed in β-thalassemia than is required for erythropoiesis and that increasing the concentration of hepcidin in the body of such patients might be therapeutic, limiting iron overload. Here we demonstrate that a moderate increase in expression of hepcidin in β-thalassemic mice limits iron overload, decreases formation of insoluble membrane-bound globins and reactive oxygen species, and improves anemia. Mice with increased hepcidin expression also demonstrated an increase in the lifespan of their red cells, reversal of ineffective erythropoiesis and splenomegaly, and an increase in total hemoglobin levels. These data led us to suggest that therapeutics that could increase hepcidin levels or act as hepcidin agonists might help treat the abnormal iron absorption in individuals with β-thalassemia and related disorders.
Figures



Comment in
-
A tincture of hepcidin cures all: the potential for hepcidin therapeutics.J Clin Invest. 2010 Dec;120(12):4187-90. doi: 10.1172/JCI45043. Epub 2010 Nov 22. J Clin Invest. 2010. PMID: 21099105 Free PMC article.
References
-
- Steinberg MH, Forget BG, Higgs DR, Nagel RL.Disorders of hemoglobin: Genetics, Pathophysiology and Clinical Management . Cambridge, United Kingdom: Cambridge University Press; 2001.
-
- Pippard MJ, Callender ST, Warner GT, Weatherall DJ. Iron absorption and loading in beta-thalassaemia intermedia. Lancet. 1979;2(8147):819–821. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

