Pathogenic T cells have a paradoxical protective effect in murine autoimmune diabetes by boosting Tregs
- PMID: 21099113
- PMCID: PMC2993590
- DOI: 10.1172/JCI42945
Pathogenic T cells have a paradoxical protective effect in murine autoimmune diabetes by boosting Tregs
Abstract
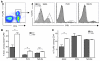
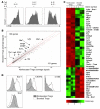
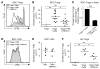
CD4+CD25+Foxp3+ Tregs play a major role in prevention of autoimmune diseases. The suppressive effect of Tregs on effector T cells (Teffs), the cells that can mediate autoimmunity, has been extensively studied. However, the in vivo impact of Teff activation on Tregs during autoimmunity has not been explored. In this study, we have shown that CD4+ Teff activation strongly boosts the expansion and suppressive activity of Tregs. This helper function of CD4+ T cells, which we believe to be novel, was observed in the pancreas and draining lymph nodes in mouse recipients of islet-specific Teffs and Tregs. Its physiological impact was assessed in autoimmune diabetes. When islet-specific Teffs were transferred alone, they induced diabetes. Paradoxically, when the same Teffs were cotransferred with islet-specific Tregs, they induced disease protection by boosting Treg expansion and suppressive function. RNA microarray analyses suggested that TNF family members were involved in the Teff-mediated Treg boost. In vivo experiments showed that this Treg boost was partially dependent on TNF but not on IL-2. This feedback regulatory loop between Teffs and Tregs may be critical to preventing or limiting the development of autoimmune diseases.
Figures

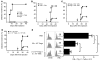


Comment in
-
Can TNF-α boost regulatory T cells?J Clin Invest. 2010 Dec;120(12):4190-2. doi: 10.1172/JCI45262. Epub 2010 Nov 22. J Clin Invest. 2010. PMID: 21099102 Free PMC article.
References
-
- Danke NA, Koelle DM, Yee C, Beheray S, Kwok WW. Autoreactive T cells in healthy individuals. . J Immunol. 2004;172(10):5967–5972. - PubMed
-
- Horwitz MS, Bradley LM, Harbertson J, Krahl T, Lee J, Sarvetnick N. Diabetes induced by Coxsackie virus: initiation by bystander damage and not molecular mimicry. Nat Med. 1998;4(7):781–785. - PubMed
-
- Cuffy MC, et al. Induction of indoleamine 2,3-dioxygenase in vascular smooth muscle cells by interferon-gamma contributes to medial immunoprivilege. J Immunol. 2007;179(8):5246–5254. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

