Vancomycin-resistant Enterococcus domination of intestinal microbiota is enabled by antibiotic treatment in mice and precedes bloodstream invasion in humans
- PMID: 21099116
- PMCID: PMC2993598
- DOI: 10.1172/JCI43918
Vancomycin-resistant Enterococcus domination of intestinal microbiota is enabled by antibiotic treatment in mice and precedes bloodstream invasion in humans
Abstract
Bloodstream infection by highly antibiotic-resistant bacteria, such as vancomycin-resistant Enterococcus (VRE), is a growing clinical problem that increasingly defies medical intervention. Identifying patients at high risk for bacterial sepsis remains an important clinical challenge. Recent studies have shown that antibiotics can alter microbial diversity in the intestine. Here, we characterized these effects using 16s rDNA pyrosequencing and demonstrated that antibiotic treatment of mice enabled exogenously administered VRE to efficiently and nearly completely displace the normal microbiota of the small and large intestine. In the clinical setting, we found that intestinal domination by VRE preceded bloodstream infection in patients undergoing allogeneic hematopoietic stem cell transplantation. Our results demonstrate that antibiotics perturb the normal commensal microbiota and set the stage for intestinal domination by bacteria associated with hospital-acquired infections. Thus, high-throughput DNA sequencing of the intestinal microbiota could identify patients at high risk of developing bacterial sepsis.
Figures


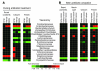
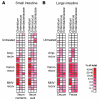
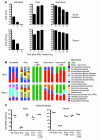

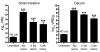

Comment in
-
Which species are in your feces?J Clin Invest. 2010 Dec;120(12):4182-5. doi: 10.1172/JCI45263. Epub 2010 Nov 22. J Clin Invest. 2010. PMID: 21099104 Free PMC article.
References
-
- Donskey CJ. Antibiotic regimens and intestinal colonization with antibiotic-resistant gram-negative bacilli. Clin Infect Dis. 2006;43(suppl 2):S62–S69. - PubMed
-
- Rice LB. Antimicrobial resistance in gram–positive bacteria. Am J Med. 2006;119(6 suppl 1):S11–S19. - PubMed
-
- Bartlett JG. Narrative review: the new epidemic of Clostridium difficile-associated enteric disease. Ann Intern Med. 2006;145(10):758–764. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

