Expression of αvβ8 integrin on dendritic cells regulates Th17 cell development and experimental autoimmune encephalomyelitis in mice
- PMID: 21099117
- PMCID: PMC2993595
- DOI: 10.1172/JCI43786
Expression of αvβ8 integrin on dendritic cells regulates Th17 cell development and experimental autoimmune encephalomyelitis in mice
Abstract
Th17 cells promote a variety of autoimmune diseases, including psoriasis, multiple sclerosis, rheumatoid arthritis, and inflammatory bowel disease. TGF-β is required for conversion of naive T cells to Th17 cells, but the mechanisms regulating this process are unknown. Integrin αvβ8 on DCs can activate TGF-β, and this process contributes to the development of induced Tregs. Here, we have now shown that integrin αvβ8 expression on DCs plays a critical role in the differentiation of Th17 cells. Th17 cells were nearly absent in the colons of mice lacking αvβ8 expression on DCs. In addition, these mice and the DCs harvested from them had an impaired ability to convert naive T cells into Th17 cells in vivo and in vitro, respectively. Importantly, mice lacking αvβ8 on DCs showed near-complete protection from experimental autoimmune encephalomyelitis. Our results therefore suggest that the integrin αvβ8 pathway is biologically important and that αvβ8 expression on DCs could be a therapeutic target for the treatment of Th17-driven autoimmune disease.
Figures

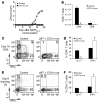



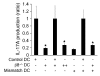
Comment in
-
Integral role of integrins in Th17 development.J Clin Invest. 2010 Dec;120(12):4185-7. doi: 10.1172/JCI45450. Epub 2010 Nov 22. J Clin Invest. 2010. PMID: 21099101 Free PMC article.
References
-
- Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24(2):179–189. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 AI024674/AI/NIAID NIH HHS/United States
- R37 HL053949/HL/NHLBI NIH HHS/United States
- HL53949/HL/NHLBI NIH HHS/United States
- U19 AI056388/AI/NIAID NIH HHS/United States
- F32 HL095314/HL/NHLBI NIH HHS/United States
- R01 HL053949/HL/NHLBI NIH HHS/United States
- U19 AI077439/AI/NIAID NIH HHS/United States
- BB/G001103/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- HL083950/HL/NHLBI NIH HHS/United States
- HL64353/HL/NHLBI NIH HHS/United States
- AI024674/AI/NIAID NIH HHS/United States
- R01 HL083950/HL/NHLBI NIH HHS/United States
- R01 AI024674/AI/NIAID NIH HHS/United States
- R01 HL064353/HL/NHLBI NIH HHS/United States
- HL095314/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

