Is ryanodine receptor phosphorylation key to the fight or flight response and heart failure?
- PMID: 21099119
- PMCID: PMC2994341
- DOI: 10.1172/JCI45251
Is ryanodine receptor phosphorylation key to the fight or flight response and heart failure?
Abstract
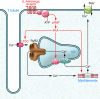
In situations of stress the heart beats faster and stronger. According to Marks and colleagues, this response is, to a large extent, the consequence of facilitated Ca²+ release from intracellular Ca²+ stores via ryanodine receptor 2 (RyR2), thought to be due to catecholamine-induced increases in RyR2 phosphorylation at serine 2808 (S2808). If catecholamine stimulation is sustained (for example, as occurs in heart failure), RyR2 becomes hyperphosphorylated and "leaky," leading to arrhythmias and other pathology. This "leaky RyR2 hypothesis" is highly controversial. In this issue of the JCI, Marks and colleagues report on two new mouse lines with mutations in S2808 that provide strong evidence supporting their theory. Moreover, the experiments revealed an influence of redox modifications of RyR2 that may account for some discrepancies in the field.
Figures

Comment on
-
Role of chronic ryanodine receptor phosphorylation in heart failure and β-adrenergic receptor blockade in mice.J Clin Invest. 2010 Dec;120(12):4375-87. doi: 10.1172/JCI37649. Epub 2010 Nov 22. J Clin Invest. 2010. PMID: 21099115 Free PMC article.
-
Phosphorylation of the ryanodine receptor mediates the cardiac fight or flight response in mice.J Clin Invest. 2010 Dec;120(12):4388-98. doi: 10.1172/JCI32726. Epub 2010 Nov 22. J Clin Invest. 2010. PMID: 21099118 Free PMC article.
References
-
- Luo W, et al. Targeted ablation of the phospholamban gene is associated with markedly enhanced myocardial contractility and loss of beta-agonist stimulation. Circ Res. 1994;75(3):401–409. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

