Biliverdin reductase A in the prevention of cellular senescence against oxidative stress
- PMID: 21099244
- PMCID: PMC3041934
- DOI: 10.3858/emm.2011.43.1.002
Biliverdin reductase A in the prevention of cellular senescence against oxidative stress
Abstract
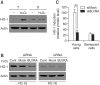
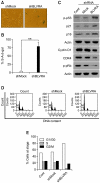
Biliverdin reductase A (BLVRA), an enzyme that converts biliverdin to bilirubin, has recently emerged as a key regulator of the cellular redox cycle. However, the role of BLVRA in the aging process remains unclear. To study the role of BLVRA in the aging process, we compared the stress responses of young and senescent human diploid fibroblasts (HDFs) to the reactive oxygen species (ROS) inducer, hydrogen peroxide (H2O2). H2O2 markedly induced BLVRA activity in young HDFs, but not in senescent HDFs. Additionally, depletion of BLVRA reduced the H2O2-dependent induction of heme oxygenase-1 (HO-1) in young HDFs, but not in senescent cells, suggesting an aging-dependent differential modulation of responses to oxidative stress. The role of BLVRA in the regulation of cellular senescence was confirmed when lentiviral RNAi- transfected stable primary HDFs with reduced BLVRA expression showed upregulation of the CDK inhibitor family members p16, p53, and p21, followed by cell cycle arrest in G0-G1 phase with high expression of senescence-associated β-galactosidase. Taken together, these data support the notion that BLVRA contributes significantly to modulation of the aging process by adjusting the cellular oxidative status.
Figures


Similar articles
-
Biliverdin Reductase A Protects Lens Epithelial Cells against Oxidative Damage and Cellular Senescence in Age-Related Cataract.Oxid Med Cell Longev. 2022 Jul 19;2022:5628946. doi: 10.1155/2022/5628946. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35910837 Free PMC article. Clinical Trial.
-
Tat-biliverdin reductase A protects INS-1 cells from human islet amyloid polypeptide-induced cytotoxicity by alleviating oxidative stress and ER stress.Cell Biol Int. 2017 May;41(5):514-524. doi: 10.1002/cbin.10750. Epub 2017 Mar 20. Cell Biol Int. 2017. PMID: 28198575
-
Reactive oxygen species promotes cellular senescence in normal human epidermal keratinocytes through epigenetic regulation of p16(INK4a.).Biochem Biophys Res Commun. 2014 Sep 26;452(3):622-8. doi: 10.1016/j.bbrc.2014.08.123. Epub 2014 Aug 30. Biochem Biophys Res Commun. 2014. PMID: 25181340
-
Redox Functions of Heme Oxygenase-1 and Biliverdin Reductase in Diabetes.Trends Endocrinol Metab. 2018 Feb;29(2):74-85. doi: 10.1016/j.tem.2017.11.005. Epub 2017 Dec 14. Trends Endocrinol Metab. 2018. PMID: 29249571 Review.
-
Replicative senescence and oxidant-induced premature senescence. Beyond the control of cell cycle checkpoints.Ann N Y Acad Sci. 2000 Jun;908:111-25. doi: 10.1111/j.1749-6632.2000.tb06640.x. Ann N Y Acad Sci. 2000. PMID: 10911952 Review.
Cited by
-
Guiqi polysaccharide protects the normal human fetal lung fibroblast WI-38 cells from H2O2-induced premature senescence.Int J Clin Exp Pathol. 2015 May 1;8(5):4398-407. eCollection 2015. Int J Clin Exp Pathol. 2015. PMID: 26191131 Free PMC article.
-
Go green: the anti-inflammatory effects of biliverdin reductase.Front Pharmacol. 2012 Mar 16;3:47. doi: 10.3389/fphar.2012.00047. eCollection 2012. Front Pharmacol. 2012. PMID: 22438844 Free PMC article.
-
Molecular modeling to provide insight into the substrate binding and catalytic mechanism of human biliverdin-IXα reductase.J Phys Chem B. 2012 Aug 16;116(32):9580-94. doi: 10.1021/jp301456j. Epub 2012 Aug 7. J Phys Chem B. 2012. PMID: 22823425 Free PMC article.
-
Physiological antioxidative network of the bilirubin system in aging and age-related diseases.Front Pharmacol. 2012 Mar 14;3:45. doi: 10.3389/fphar.2012.00045. eCollection 2012. Front Pharmacol. 2012. PMID: 22457648 Free PMC article.
-
Thymoquinone increases the expression of neuroprotective proteins while decreasing the expression of pro-inflammatory cytokines and the gene expression NFκB pathway signaling targets in LPS/IFNγ -activated BV-2 microglia cells.J Neuroimmunol. 2018 Jul 15;320:87-97. doi: 10.1016/j.jneuroim.2018.04.018. Epub 2018 May 4. J Neuroimmunol. 2018. PMID: 29759145 Free PMC article.
References
-
- Aggeli IK, Gaitanaki C, Beis I. Involvement of JNKs and p38-MAPK/MSK1 pathways in H2O2-induced upregulation of heme oxygenase-1 mRNA in H9c2 cells. Cell Signal. 2006;18:1801–1812. - PubMed
-
- Ahmad Z, Salim M, Maines MD. Human biliverdin reductase is a leucine zipper-like DNA-binding protein and functions in transcriptional activation of heme oxygenase-1 by oxidative stress. J Biol Chem. 2002;277:9226–9232. - PubMed
-
- Beale SI, Cornejo J. Enzymatic heme oxygenase activity in soluble extracts of the unicellular red alga, Cyanidium caldarium. Arch Biochem Biophys. 1984;235:371–384. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
