No non-redundant function of suppressor of cytokine signaling 2 in insulin producing β-cells
- PMID: 21099320
- PMCID: PMC3322539
- DOI: 10.4161/isl.2.4.12556
No non-redundant function of suppressor of cytokine signaling 2 in insulin producing β-cells
Abstract
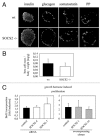
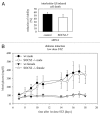
The members of the Suppressor of Cytokine Signaling (SOCS) protein family mainly modulate the Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway. SOCS-1 and SOCS-3 have already been shown to influence growth and apoptosis of pancreatic beta cells. We hypothesized that SOCS-2, which is expressed in pancreatic islets, also contributes to β-cell physiology. We tested this hypothesis in vivo in SOCS-2-/- knockout mice and in vitro in Ins-1E rat insulinoma cells. We found that SOCS-2-/- mice have normal islet insulin secretion and unchanged glucose and insulin tolerance compared to wildtype controls. SOCS-2-/- are bigger than wildtype mice but body weight-corrected β-cell mass and islet morphology were normal. Growth hormone-induced proliferation of Ins-1E cells was not affected by either siRNA-mediated SOCS-2 knockdown or stable SOCS-2 overexpression. Interleukin-1β mediated cell death in vitro was unchanged after SOCS-2 knockdown. Similarly, autoimmune destruction of beta cells in vivo after multiple low-dose injections of streptozotocin (STZ) was not altered in SOCS-2-/- mice. In summary, SOCS-2-/- knockout mice have a normal function of insulin-producing pancreatic β-cells, a fully adapted beta cell mass and a normal morphology of the endocrine islets. Based on in vitro evidence, the increased β-cell mass in the mutants is likely due to indirect adaptive mechanisms and not the result of altered growth hormone signaling within the β-cells. Immune mediated β-cell destruction is also not affected by SOCS-2 ablation in vitro and in vivo.
Figures


References
-
- Rico-Bautista E, Flores-Morales A, Fernandez-Perez L. Suppressor of cytokine signaling (SOCS) 2, a protein with multiple functions. Cytokine Growth Factor Rev. 2006;17:431–439. - PubMed
-
- Cottet S, Dupraz P, Hamburger F, Dolci W, Jaquet M, Thorens B. SOCS-1 protein prevents Janus Kinase/STAT-dependent inhibition of beta cell insulin gene transcription and secretion in response to interferongamma. J Biol Chem. 2001;276:25862–25870. - PubMed
-
- Karlsen AE, Heding PE, Frobose H, Ronn SG, Kruhoffer M, Orntoft TF, et al. Suppressor of cytokine signalling (SOCS)-3 protects beta cells against IL-1beta-mediated toxicity through inhibition of multiple nuclear factor-kappaB-regulated proapoptotic pathways. Diabetologia. 2004;47:1998–2011. - PubMed
-
- Chong MM, Chen Y, Darwiche R, Dudek NL, Irawaty W, Santamaria P, et al. Suppressor of cytokine signaling-1 overexpression protects pancreatic beta cells from CD8+ T cell-mediated autoimmune destruction. J Immunol. 2004;172:5714–5721. - PubMed
-
- Lindberg K, Ronn SG, Tornehave D, Richter H, Hansen JA, Romer J, et al. Regulation of pancreatic beta-cell mass and proliferation by SOCS-3. J Mol Endocrinol. 2005;35:231–243. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
