CSN complex controls the stability of selected synaptic proteins via a torsinA-dependent process
- PMID: 21102408
- PMCID: PMC3020110
- DOI: 10.1038/emboj.2010.285
CSN complex controls the stability of selected synaptic proteins via a torsinA-dependent process
Abstract
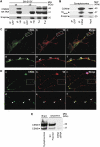
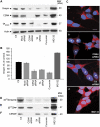
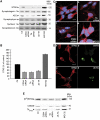
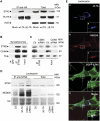
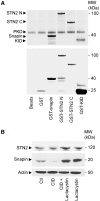
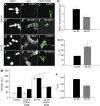
DYT1 dystonia is caused by an autosomal dominant mutation that leads to a glutamic acid deletion in torsinA (TA), a member of the AAA+ ATPase superfamily. In this study, we identified a novel-binding partner of TA, the subunit 4 (CSN4) of CSN signalosome. TA binds CSN4 and the synaptic regulator snapin in neuroblastoma cells and in brain synaptosomes. CSN4 and TA are required for the stability of both snapin and the synaptotagmin-specific endocytic adaptor stonin 2, as downregulation of CSN4 or TA reduces the levels of both proteins. Snapin is phosphorylated by the CSN-associated kinase protein kinase D (PKD) and its expression is decreased upon PKD inhibition. In contrast, the stability of stonin 2 is regulated by neddylation, another CSN-associated activity. Overexpression of the pathological TA mutant (ΔE-TA) reduces stonin 2 expression, causing the accumulation of the calcium sensor synaptotagmin 1 on the cell surface. Retrieval of surface-stranded synaptotagmin 1 is restored by overexpression of stonin 2 in ΔE-TA-expressing cells, suggesting that the DYT1 mutation compromises the role of TA in protein stabilisation and synaptic vesicle recycling.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Similar articles
-
The dystonia-associated protein torsinA modulates synaptic vesicle recycling.J Biol Chem. 2008 Mar 21;283(12):7568-79. doi: 10.1074/jbc.M704097200. Epub 2007 Dec 31. J Biol Chem. 2008. PMID: 18167355
-
Molecular basis of synaptic vesicle cargo recognition by the endocytic sorting adaptor stonin 2.J Cell Biol. 2007 Dec 31;179(7):1497-510. doi: 10.1083/jcb.200708107. J Cell Biol. 2007. PMID: 18166656 Free PMC article.
-
UNC-41/stonin functions with AP2 to recycle synaptic vesicles in Caenorhabditis elegans.PLoS One. 2012;7(7):e40095. doi: 10.1371/journal.pone.0040095. Epub 2012 Jul 10. PLoS One. 2012. PMID: 22808098 Free PMC article.
-
Stonins--specialized adaptors for synaptic vesicle recycling and beyond?Traffic. 2010 Jan;11(1):8-15. doi: 10.1111/j.1600-0854.2009.00971.x. Traffic. 2010. PMID: 19732400 Review.
-
TorsinA and DYT1 dystonia: a synaptopathy?Biochem Soc Trans. 2010 Apr;38(2):452-6. doi: 10.1042/BST0380452. Biochem Soc Trans. 2010. PMID: 20298201 Review.
Cited by
-
Update on pediatric dystonias: etiology, epidemiology, and management.Degener Neurol Neuromuscul Dis. 2012 Apr 11;2:29-41. doi: 10.2147/DNND.S16082. eCollection 2012. Degener Neurol Neuromuscul Dis. 2012. PMID: 30890876 Free PMC article. Review.
-
Regulation of Cop9 signalosome activity by the EF-hand Ca2+-binding protein tescalcin.J Cell Sci. 2014 Jun 1;127(Pt 11):2448-59. doi: 10.1242/jcs.139592. Epub 2014 Mar 21. J Cell Sci. 2014. PMID: 24659803 Free PMC article.
-
Decreased dopamine receptor 1 activity and impaired motor-skill transfer in Dyt1 ΔGAG heterozygous knock-in mice.Behav Brain Res. 2015 Feb 15;279:202-10. doi: 10.1016/j.bbr.2014.11.037. Epub 2014 Nov 29. Behav Brain Res. 2015. PMID: 25451552 Free PMC article.
-
DYT-TOR1A dystonia: an update on pathogenesis and treatment.Front Neurosci. 2023 Aug 10;17:1216929. doi: 10.3389/fnins.2023.1216929. eCollection 2023. Front Neurosci. 2023. PMID: 37638318 Free PMC article. Review.
-
Functional Genomic Analyses of Mendelian and Sporadic Disease Identify Impaired eIF2α Signaling as a Generalizable Mechanism for Dystonia.Neuron. 2016 Dec 21;92(6):1238-1251. doi: 10.1016/j.neuron.2016.11.012. Epub 2016 Dec 8. Neuron. 2016. PMID: 27939583 Free PMC article.
References
-
- Balcioglu A, Kim MO, Sharma N, Cha JH, Breakefield XO, Standaert DG (2007) Dopamine release is impaired in a mouse model of DYT1 dystonia. J Neurochem 102: 783–788 - PubMed
-
- Bech-Otschir D, Seeger M, Dubiel W (2002) The COP9 signalosome: at the interface between signal transduction and ubiquitin-dependent proteolysis. J Cell Sci 115: 467–473 - PubMed
-
- Breakefield XO, Kamm C, Hanson PI (2001) TorsinA: movement at many levels. Neuron 31: 9–12 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

