AKAP-Lbc enhances cyclic AMP control of the ERK1/2 cascade
- PMID: 21102438
- PMCID: PMC3042953
- DOI: 10.1038/ncb2130
AKAP-Lbc enhances cyclic AMP control of the ERK1/2 cascade
Abstract
Mitogen-activated protein kinase (MAPK) cascades propagate a variety of cellular activities. Processive relay of signals through RAF-MEK-ERK modulates cell growth and proliferation. Signalling through this ERK cascade is frequently amplified in cancers, and drugs such as sorafenib (which is prescribed to treat renal and hepatic carcinomas) and PLX4720 (which targets melanomas) inhibit RAF kinases. Natural factors that influence ERK1/2 signalling include the second messenger cyclic AMP. However, the mechanisms underlying this cascade have been difficult to elucidate. We demonstrate that the A-kinase-anchoring protein AKAP-Lbc and the scaffolding protein kinase suppressor of Ras (KSR-1) form the core of a signalling network that efficiently relay signals from RAF, through MEK, and on to ERK1/2. AKAP-Lbc functions as an enhancer of ERK signalling by securing RAF in the vicinity of MEK1 and synchronizing protein kinase A (PKA)-mediated phosphorylation of Ser 838 on KSR-1. This offers mechanistic insight into cAMP-responsive control of ERK signalling events.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

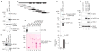


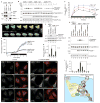
References
-
- Raman M, Chen W, Cobb MH. Differential regulation and properties of MAPKs. Oncogene. 2007;26:3100–3112. - PubMed
-
- Mansour SJ, et al. Transformation of mammalian cells by constitutively active MAP kinase kinase. Science. 1994;265:966–970. - PubMed
-
- Wan PT, et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell. 2004;116:855–867. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

