A cytoplasmic dynein tail mutation impairs motor processivity
- PMID: 21102439
- PMCID: PMC3385513
- DOI: 10.1038/ncb2127
A cytoplasmic dynein tail mutation impairs motor processivity
Abstract
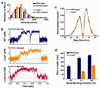
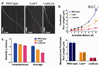
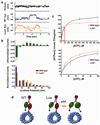
Mutations in the tail of the cytoplasmic dynein molecule have been reported to cause neurodegenerative disease in mice. The mutant mouse strain Legs at odd angles (Loa) has impaired retrograde axonal transport, but the molecular deficiencies in the mutant dynein molecule, and how they contribute to neurodegeneration, are unknown. To address these questions, we purified dynein from wild-type mice and the Legs at odd angles mutant mice. Using biochemical, single-molecule, and live-cell-imaging techniques, we find a marked inhibition of motor run-length in vitro and in vivo, and significantly altered motor domain coordination in the dynein from mutant mice. These results suggest a potential role for the dynein tail in motor function, and provide direct evidence for a link between single-motor processivity and disease.
Figures


Comment in
-
Dynein at odd angles?Nat Cell Biol. 2010 Dec;12(12):1126-8. doi: 10.1038/ncb1210-1126. Epub 2010 Nov 21. Nat Cell Biol. 2010. PMID: 21102436
References
-
- Paschal BM, Vallee RB. Retrograde transport by the microtubule associated protein MAP 1C. Nature. 1987;330:181–183. - PubMed
-
- Hafezparast M, et al. Mutations in dynein link motor neuron degeneration to defects in retrograde transport. Science. 2003;300:808–812. - PubMed
-
- Puls I, et al. Mutant dynactin in motor neuron disease. Nat Genet. 2003;33:455–456. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

