Tissue elongation requires oscillating contractions of a basal actomyosin network
- PMID: 21102441
- PMCID: PMC3056411
- DOI: 10.1038/ncb2124
Tissue elongation requires oscillating contractions of a basal actomyosin network
Abstract
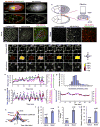
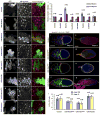
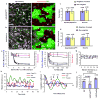
Understanding how molecular dynamics leads to cellular behaviours that ultimately sculpt organs and tissues is a major challenge not only in basic developmental biology but also in tissue engineering and regenerative medicine. Here we use live imaging to show that the basal surfaces of Drosophila follicle cells undergo a series of directional, oscillating contractions driven by periodic myosin accumulation on a polarized actin network. Inhibition of the actomyosin contractions or their coupling to extracellular matrix (ECM) blocked elongation of the whole tissue, whereas enhancement of the contractions exaggerated it. Myosin accumulated in a periodic manner before each contraction and was regulated by the small GTPase Rho, its downstream kinase, ROCK, and cytosolic calcium. Disrupting the link between the actin cytoskeleton and the ECM decreased the amplitude and period of the contractions, whereas enhancing cell-ECM adhesion increased them. In contrast, disrupting cell-cell adhesions resulted in loss of the actin network. Our findings reveal a mechanism controlling organ shape and an experimental model for the study of the effects of oscillatory actomyosin activity within a coherent cell sheet.
Figures





References
-
- Lecuit T, Lenne PF. Cell surface mechanics and the control of cell shape, tissue patterns and morphogenesis. Nat Rev Mol Cell Biol. 2007;8:633–44. - PubMed
-
- Solon J, Kaya-Copur A, Colombelli J, Brunner D. Pulsed forces timed by a ratchet-like mechanism drive directed tissue movement during dorsal closure. Cell. 2009;137:1331–42. - PubMed
-
- Bertet C, Sulak L, Lecuit T. Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature. 2004;429:667–71. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

