Conversion of vascular endothelial cells into multipotent stem-like cells
- PMID: 21102460
- PMCID: PMC3209716
- DOI: 10.1038/nm.2252
Conversion of vascular endothelial cells into multipotent stem-like cells
Erratum in
- Nat Med. 2011 Apr;17(4):514
Abstract
Mesenchymal stem cells can give rise to several cell types, but varying results depending on isolation methods and tissue source have led to controversies about their usefulness in clinical medicine. Here we show that vascular endothelial cells can transform into multipotent stem-like cells by an activin-like kinase-2 (ALK2) receptor-dependent mechanism. In lesions from individuals with fibrodysplasia ossificans progressiva (FOP), a disease in which heterotopic ossification occurs as a result of activating ALK2 mutations, or from transgenic mice expressing constitutively active ALK2, chondrocytes and osteoblasts expressed endothelial markers. Lineage tracing of heterotopic ossification in mice using a Tie2-Cre construct also suggested an endothelial origin of these cell types. Expression of constitutively active ALK2 in endothelial cells caused endothelial-to-mesenchymal transition and acquisition of a stem cell-like phenotype. Similar results were obtained by treatment of untransfected endothelial cells with the ligands transforming growth factor-β2 (TGF-β2) or bone morphogenetic protein-4 (BMP4) in an ALK2-dependent manner. These stem-like cells could be triggered to differentiate into osteoblasts, chondrocytes or adipocytes. We suggest that conversion of endothelial cells to stem-like cells may provide a new approach to tissue engineering.
Figures
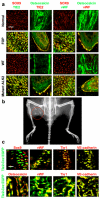
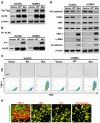




Comment in
-
Building bone from blood vessels.Nat Med. 2010 Dec;16(12):1373-4. doi: 10.1038/nm1210-1373. Nat Med. 2010. PMID: 21135844 No abstract available.
-
Transition of endothelium to cartilage and bone.Cell Stem Cell. 2011 Jan 7;8(1):10-1. doi: 10.1016/j.stem.2010.12.004. Cell Stem Cell. 2011. PMID: 21211778
References
-
- Akhurst RJ, Derynck R. TGF-beta signaling in cancer - a double-edged sword. Trends Cell Biol. 2001;11:S44–S51. - PubMed
-
- Thiery JP. Epithelial-mesenchymal transitions in tumor progression. Nat. Rev. Cancer. 2002;2:442–454. - PubMed
-
- Thiery JP. Epithelial-mesenchymal transitions in development and pathologies. Curr. Opin. Cell Biol. 2003;15:740–746. - PubMed
-
- Hay ED. The mesenchymal cell, its role in the embryo, and the remarkable signaling mechanisms that create it. Dev. Dyn. 2005;233:706–720. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

