Tanshinone IIA attenuates angiotensin II-induced apoptosis via Akt pathway in neonatal rat cardiomyocytes
- PMID: 21102479
- PMCID: PMC4002950
- DOI: 10.1038/aps.2010.176
Tanshinone IIA attenuates angiotensin II-induced apoptosis via Akt pathway in neonatal rat cardiomyocytes
Abstract
Aim: to examine the effects of tanshinone IIA, the main effective component of Salvia miltiorrhiza (known as 'Danshen' in traditional Chinese medicine) on angiotensin II (Ang II)-mediated cardiomyocyte apoptosis.
Methods: rat neonatal cardiomyocytes were primarily cultured with Ang II or Ang II plus tanshinone IIA. Myocyte apoptosis was evaluated by caspase-3 activity and DNA strand break level with TdT-mediated dUTP nick-end labeling (TUNEL) staining. Western blot analysis was employed to determine the related protein expression and flow cytometry assay was used to determine the TUNEL positive cells and the intracellular reactive oxygen species (ROS) production. SiRNA targeted to Akt was used.
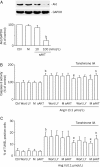
Results: ang II (0.1 micromol/L) remarkably increased caspase-3 activity, TUNEL positive cells, and cleaved caspase-3 and cytochrome c expression, but reduced Bcl-X(L) expression. These effects were effectively antagonized by pretreatment with tanshione IIA (1-3 micromol/L). Tanshinone IIA had no effect on basal ROS level, while attenuated the ROS production by Ang II. Interestingly, tanshione IIA significantly increased the phosphorylated Akt level, which was countered by the PI3K antagonist wortmannin or LY294002. Knockdown of Akt with Akt siRNA significantly reduced Akt protein levels and tanshinone IIA protective effect.
Conclusion: tanshinone IIA prevents Ang II-induced apoptosis, thereby suggesting that tanshinone IIA may be used for the prevention of the cardiac remodeling process.
Figures




References
-
- Masri C, Chandrashekhar Y. Apoptosis: a potentially reversible, meta-stable state of the heart. Heart Fail Rev. 2008;13:175–9. - PubMed
-
- Sorescu D, Griendling KK. Reactive oxygen species, mitochondria, and NAD(P)H oxidases in the development and progression of heart failure. Congest Heart Fail. 2002;8:132–40. - PubMed
-
- Paul M, Poyan Mehr A, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev. 2006;86:747–803. - PubMed
-
- Schroder D, Heger J, Piper HM, Euler G. Angiotensin II stimulates apoptosis via TGF-beta1 signaling in ventricular cardiomyocytes of rat. J Mol Med. 2006;84:975–83. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

