Kinetochore-microtubule interactions: steps towards bi-orientation
- PMID: 21102558
- PMCID: PMC3018795
- DOI: 10.1038/emboj.2010.294
Kinetochore-microtubule interactions: steps towards bi-orientation
Abstract
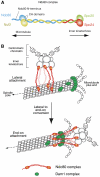
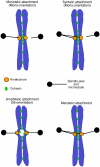
Eukaryotic cells segregate their chromosomes accurately to opposite poles during mitosis, which is necessary for maintenance of their genetic integrity. This process mainly relies on the forces generated by kinetochore-microtubule (KT-MT) attachment. During prometaphase, the KT initially interacts with a single MT extending from a spindle pole and then moves towards a spindle pole. Subsequently, MTs from the other spindle pole also interact with the KT. Eventually, one sister KT becomes attached to MTs from one pole while the other sister to those from the other pole (sister KT bi-orientation). If sister KTs interact with MTs with aberrant orientation, this must be corrected to attain proper bi-orientation (error correction) before the anaphase is initiated. Here, I discuss how KTs initially interact with MTs and how this interaction develops into bi-orientation; both processes are fundamentally crucial for proper chromosome segregation in the subsequent anaphase.
Conflict of interest statement
The author declares that he has no conflict of interest.
Figures

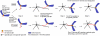

References
-
- Andrews PD, Ovechkina Y, Morrice N, Wagenbach M, Duncan K, Wordeman L, Swedlow JR (2004) Aurora B regulates MCAK at the mitotic centromere. Dev Cell 6: 253–268 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

