Asymmetric cell division: recent developments and their implications for tumour biology
- PMID: 21102610
- PMCID: PMC3941022
- DOI: 10.1038/nrm3010
Asymmetric cell division: recent developments and their implications for tumour biology
Abstract
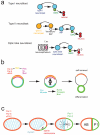
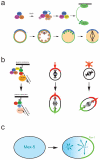
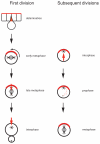
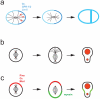
The ability of cells to divide asymmetrically is essential for generating diverse cell types during development. The past 10 years have seen tremendous progress in our understanding of this important biological process. We have learned that localized phosphorylation events are responsible for the asymmetric segregation of cell fate determinants in mitosis and that centrosomes and microtubules play important parts in this process. The relevance of asymmetric cell division for stem cell biology has added a new dimension to the field, and exciting connections between asymmetric cell division and tumorigenesis have begun to emerge.
Figures
References
-
- Conklin EG. The organization and cell-lineage of the ascidian egg. J. Acad. Nat. Sci. Philadelphia. 1905;13:1–119.
-
- Rhyu MS, Jan LY, Jan YN. Asymmetric distribution of numb protein during division of the sensory organ precursor cell confers distinct fates to daughter cells. Cell. 1994;76:477–491. - PubMed
-
- Spana EP, Kopczynski C, Goodman CS, Doe CQ. Asymmetric localization of numb autonomously determines sibling neuron identity in the Drosophila CNS. Development. 1995;121:3489–3494. - PubMed
-
- Uemura T, Shepherd S, Ackerman L, Jan LY, Jan YN. numb, a gene required in determination of cell fate during sensory organ formation in Drosophila embryos. Cell. 1989;58:349–360. - PubMed
-
- Kemphues KJ, Priess JR, Morton DG, Cheng NS. Identification of genes required for cytoplasmic localization in early C. elegans embryos. Cell. 1988;52:311–320. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

