Why do viruses cause cancer? Highlights of the first century of human tumour virology
- PMID: 21102637
- PMCID: PMC3718018
- DOI: 10.1038/nrc2961
Why do viruses cause cancer? Highlights of the first century of human tumour virology
Abstract
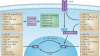
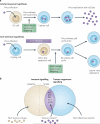
The year 2011 marks the centenary of Francis Peyton Rous's landmark experiments on an avian cancer virus. Since then, seven human viruses have been found to cause 10-15% of human cancers worldwide. Viruses have been central to modern cancer research and provide profound insights into both infectious and non-infectious cancer causes. This diverse group of viruses reveals unexpected connections between innate immunity, immune sensors and tumour suppressor signalling that control both viral infection and cancer. This Timeline article describes common features of human tumour viruses and discusses how new technologies can be used to identify infectious causes of cancer.
Figures


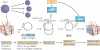
References
-
- Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int. J. Cancer. 2006;118:3030–3044. - PubMed
-
- Bouvard V, et al. A review of human carcinogens-part B: biological agents. Lancet Oncol. 2009;10:321–322. - PubMed
-
- Chang MH, et al. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. Taiwan Childhood Hepatoma Study Group. N. Engl. J. Med. 1997;336:1855–1859. - PubMed
-
- Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J. Viral Hepat. 2004;11:97–107. - PubMed
-
- Goldie SJ, et al. Projected clinical benefits and cost-effectiveness of a human papillomavirus 16/18 vaccine. J. Natl Cancer Inst. 2004;96:604–615. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

