Efficacy and Safety of Doxepin 1 mg and 3 mg in a 12-week Sleep Laboratory and Outpatient Trial of Elderly Subjects with Chronic Primary Insomnia
- PMID: 21102997
- PMCID: PMC2954705
- DOI: 10.1093/sleep/33.11.1553
Efficacy and Safety of Doxepin 1 mg and 3 mg in a 12-week Sleep Laboratory and Outpatient Trial of Elderly Subjects with Chronic Primary Insomnia
Abstract
Study objectives: to evaluate the efficacy and safety of doxepin 1 mg and 3 mg in elderly subjects with chronic primary insomnia.
Design and methods: the study was a randomized, double-blind, parallel-group, placebo-controlled trial. Subjects meeting DSM-IV-TR criteria for primary insomnia were randomized to 12 weeks of nightly treatment with doxepin (DXP) 1 mg (n = 77) or 3 mg (n = 82), or placebo (PBO; n = 81). Efficacy was assessed using polysomnography (PSG), patient reports, and clinician ratings. Objective efficacy data are reported for Nights (N) 1, 29, and 85; subjective efficacy data during Weeks 1, 4, and 12; and Clinical Global Impression (CGI) scale and Patient Global Impression (PGI) scale data after Weeks 2, 4, and 12 of treatment. Safety assessments were conducted throughout the study.
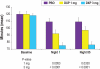
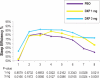
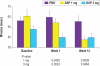
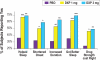
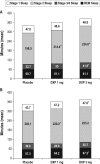
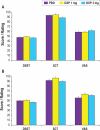
Results: DXP 3 mg led to significant improvement versus PBO on N1 in wake time after sleep onset (WASO; P < 0.0001; primary endpoint), total sleep time (TST; P < 0.0001), overall sleep efficiency (SE; P < 0.0001), SE in the last quarter of the night (P < 0.0001), and SE in Hour 8 (P < 0.0001). These improvements were sustained at N85 for all variables, with significance maintained for WASO, TST, overall SE, and SE in the last quarter of the night. DXP 3 mg significantly improved patient-reported latency to sleep onset (Weeks 1, 4, and 12), subjective TST (Weeks 1, 4, and 12), and sleep quality (Weeks 1, 4, and 12). Several global outcome-related variables were significantly improved, including the severity and improvement items of the CGI (Weeks 2, 4, and 12), and all 5 items of the PGI (Week 12; 4 items after Weeks 2 and 4). Significant improvements were observed for DXP 1 mg for several measures including WASO, TST, overall SE, and SE in the last quarter of the night at several time points. Rates of discontinuation were low, and the safety profiles were comparable across the 3 treatment groups. There were no significant next-day residual effects; additionally, there were no reports of memory impairment, complex sleep behaviors, anticholinergic effects, weight gain, or increased appetite.
Conclusions: DXP 1 mg and 3 mg administered nightly to elderly chronic insomnia patients for 12 weeks resulted in significant and sustained improvements in most endpoints. These improvements were not accompanied by evidence of next-day residual sedation or other significant adverse effects. DXP also demonstrated improvements in both patient- and physician-based ratings of global insomnia outcome. The efficacy of DXP at the doses used in this study is noteworthy with respect to sleep maintenance and early morning awakenings given that these are the primary sleep complaints of the elderly. This study, the longest placebo-controlled, double-blind, polysomnographic trial of nightly pharmacotherapy for insomnia in the elderly, provides the best evidence to date of the sustained efficacy and safety of an insomnia medication in older adults.
Keywords: Chronic insomnia; early morning awakenings; elderly adults; sleep maintenance insomnia; wake time after sleep onset.
Figures

References
-
- American Psychiatric Association: Diagnostic and statistical manual of mental disorders, fourth edition, text revision. Washington, DC: American Psychiatric Association; 2000. pp. 599–604.
-
- Lichstein KL, Durrence HH, Taylor DJ, et al. Epidemiology of sleep: age, gender, and ethnicity. Mahwah, NJ: Erlbam; 2004.
-
- Drake CL, Roehrs T, Roth T. Insomnia causes, consequences, and therapeutics: an overview. Depress Anxiety. 2003;18:163–76. - PubMed
-
- Janson C, Lindberg E, Gislason T, Elmasry A, Boman G. Insomnia in men-a 10-year prospective population based study. Sleep. 2001;24:425–30. - PubMed
-
- Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6:97–111. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

