A Direct Comparison of the Anticancer Activities of Digitoxin MeON-Neoglycosides and O-Glycosides: Oligosaccharide Chain Length-Dependent Induction of Caspase-9-Mediated Apoptosis
- PMID: 21103068
- PMCID: PMC2916185
- DOI: 10.1021/ml1000933
A Direct Comparison of the Anticancer Activities of Digitoxin MeON-Neoglycosides and O-Glycosides: Oligosaccharide Chain Length-Dependent Induction of Caspase-9-Mediated Apoptosis
Abstract
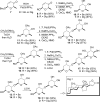
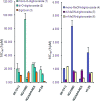
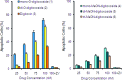
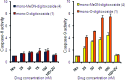
Digitoxin is a cardiac glycoside currently being investigated for potential use in oncology. While a number of structure-activity relationship studies have been conducted, an investigation of anticancer activity as a function of oligosaccharide chain length has not yet been performed. We generated mono-, di-, and tri-O-digitoxoside derivatives of digitoxin and compared their activity to the corresponding MeON-neoglycosides. Both classes of cardenolide derivatives display comparable oligosaccharide chain length-dependent cytotoxicity toward human cancer cell lines. Further investigation revealed that both classes of compounds induce caspase-9-mediated apoptosis in non-small cell lung cancer cells (NCI-H460). Since O-glycosides and MeON-neoglycosides share a similar mode of action, the convenience of MeON-neoglycosylation could be exploited in future SAR work to rapidly survey large numbers of carbohydrates to prioritize selected O-glycoside candidates for traditional synthesis.
Figures



Similar articles
-
Synthesis of MeON-neoglycosides of digoxigenin with 6-deoxy- and 2,6-dideoxy-d-glucose derivatives and their anticancer activity.Bioorg Med Chem Lett. 2017 Aug 1;27(15):3359-3364. doi: 10.1016/j.bmcl.2017.06.008. Epub 2017 Jun 3. Bioorg Med Chem Lett. 2017. PMID: 28633895
-
Synthesis of C3-Neoglycosides of digoxigenin and their anticancer activities.Eur J Med Chem. 2018 Feb 10;145:252-262. doi: 10.1016/j.ejmech.2017.12.086. Epub 2018 Jan 2. Eur J Med Chem. 2018. PMID: 29329000
-
Synthesis of MeON-Glycoside Derivatives of Oleanolic Acid by Neoglycosylation and Evaluation of Their Cytotoxicity Against Selected Cancer Cell Lines.Molecules. 2021 Feb 2;26(3):772. doi: 10.3390/molecules26030772. Molecules. 2021. PMID: 33540945 Free PMC article.
-
O-Glycoside quercetin derivatives: Biological activities, mechanisms of action, and structure-activity relationship for drug design, a review.Phytother Res. 2022 Feb;36(2):778-807. doi: 10.1002/ptr.7352. Epub 2021 Dec 29. Phytother Res. 2022. PMID: 34964515 Review.
-
Digitoxin as an anticancer agent with selectivity for cancer cells: possible mechanisms involved.Expert Opin Ther Targets. 2007 Aug;11(8):1043-53. doi: 10.1517/14728222.11.8.1043. Expert Opin Ther Targets. 2007. PMID: 17665977 Review.
Cited by
-
Stereoselective glycosylation reactions with 2-deoxyglucose: a computational study of some catalysts.Comput Theor Chem. 2023 Jun;1224:114122. doi: 10.1016/j.comptc.2023.114122. Epub 2023 Apr 6. Comput Theor Chem. 2023. PMID: 37214423 Free PMC article.
-
Na+/K+-ATPase Revisited: On Its Mechanism of Action, Role in Cancer, and Activity Modulation.Molecules. 2021 Mar 28;26(7):1905. doi: 10.3390/molecules26071905. Molecules. 2021. PMID: 33800655 Free PMC article. Review.
-
Cardiac glycoside activities link Na(+)/K(+) ATPase ion-transport to breast cancer cell migration via correlative SAR.ACS Chem Biol. 2015 Feb 20;10(2):561-9. doi: 10.1021/cb500665r. Epub 2014 Nov 26. ACS Chem Biol. 2015. PMID: 25334087 Free PMC article.
-
Phenanthroline-Assisted Stereoselective Synthesis of 2-Deoxy Glycosides.ACS Omega. 2025 Apr 29;10(18):18700-18708. doi: 10.1021/acsomega.5c00189. eCollection 2025 May 13. ACS Omega. 2025. PMID: 40385149 Free PMC article.
-
Monosaccharide digitoxin derivative sensitize human non-small cell lung cancer cells to anoikis through Mcl-1 proteasomal degradation.Biochem Pharmacol. 2014 Mar 1;88(1):23-35. doi: 10.1016/j.bcp.2013.10.027. Epub 2013 Nov 11. Biochem Pharmacol. 2014. PMID: 24231508 Free PMC article.
References
-
- Kaplan J. H. Biochemistry of Na,K-ATPase. Annu. Rev. Biochem. 2002, 71, 511–535. - PubMed
-
- Haux J. Digitalis; impinges on more than just the (ion-) pump. Med. Hypotheses 2002, 59, 781–782. - PubMed
-
- Stenkvist B. Cardenolides and cancer. Anti-Cancer Drugs 2001, 12, 635–636. - PubMed
-
- Newman R. A.; Yang P.; Pawlus A. D.; Block K. I. Cardiac Glycosides as Novel Cancer Therapeutic Agents. Mol. Interventions 2008, 8, 36–49. - PubMed
-
- Lopez-Lazaro M.; Pastor N.; Azrak S. S.; Ayuso M. J.; Austin C. A.; Cortes F. Digitoxin inhibits the growth of cancer cell lines at concentrations commonly found in cardiac patients. J. Nat. Prod. 2005, 68, 1642–1645, and references cited therein. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

