Fluorouracil cream 0.5% for the treatment of actinic keratoses on the face and anterior scalp: interim results of an 18-month open-label study
- PMID: 21103318
- PMCID: PMC2989824
Fluorouracil cream 0.5% for the treatment of actinic keratoses on the face and anterior scalp: interim results of an 18-month open-label study
Abstract
Objective: This study further assessed the long-term safety and efficacy of fluorouracil cream 0.5% in patients with multiple actinic keratosis (AK) on the face/anterior scalp and other body sites.
Design/setting: This 18-month, prospective, open-label, multicenter study comprised two treatment cycles separated by 12 months. Cycle 1 included treatment of AK lesions on the face, anterior scalp, posterior scalp, ears, neck, lips, arms, and/or hands. Once-daily fluorouracil cream 0.5% was applied for four weeks as tolerated, followed by four weeks of follow-up in each treatment cycle.
Participants: Adults (N=277) with five or more visible and/or palpable AK lesions on the face/anterior scalp and five or more lesions on the posterior scalp, ears, neck, lips, arms, and/or hands were enrolled.
Measurements: Main outcome measures included adverse events (AEs) and reduction/clearance of AK lesions on the face/anterior scalp after four weeks of treatment.
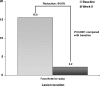
Results: RESULTS for treatment of AK lesions on the face/anterior scalp for Cycle 1 are reported. All 277 patients were treated during Cycle 1. Besides anticipated application-site reactions (67.9% and 19.1% of patients experiencing mild-to-moderate and severe events, respectively) and eye irritation, overall incidence of treatment-emergent AEs was low. No individual AE appeared in greater than four percent of patients. At the end of Cycle 1, significant reductions were noted in lesion counts on the face/anterior scalp (84.8%; P<0.0001). Clearance rate for lesions on the face and anterior scalp was 39.8 percent at eight weeks.
Conclusion: RESULTS indicate that fluorouracil cream 0.5% is safe and effective for patients with multiple AK lesions on the face/anterior scalp.
Figures
Similar articles
-
Fluorouracil cream 0.5% for actinic keratoses on multiple body sites: an 18-month open-label study.Cutis. 2010 May;85(5):267-73. Cutis. 2010. PMID: 20540418 Clinical Trial.
-
Evaluation of the efficacy and tolerability of 0.5% fluorouracil cream and 5% fluorouracil cream applied to each side of the face in patients with actinic keratosis.Clin Ther. 2002 Jun;24(6):990-1000. doi: 10.1016/s0149-2918(02)80012-1. Clin Ther. 2002. PMID: 12117087 Clinical Trial.
-
Imiquimod 2.5% and 3.75% for the treatment of actinic keratoses: two phase 3, multicenter, randomized, double-blind, placebo-controlled studies.J Drugs Dermatol. 2014 Feb;13(2):166-9. J Drugs Dermatol. 2014. PMID: 24509967 Clinical Trial.
-
5-fluorouracil 0.5% cream for multiple actinic or solar keratoses of the face and anterior scalp.Skin Therapy Lett. 2001 Jun;6(9):1-4. Skin Therapy Lett. 2001. PMID: 11550079 Review.
-
One-week treatment with once-daily fluorouracil cream 0.5% in participants with actinic keratoses.Cutis. 2008 Jun;81(6):509-16. Cutis. 2008. PMID: 18666394 Review.
Cited by
-
Patient-reported outcomes of topical therapies in actinic keratosis: A systematic review.Dermatol Ther. 2021 Mar;34(2):e14833. doi: 10.1111/dth.14833. Epub 2021 Feb 21. Dermatol Ther. 2021. PMID: 33527673 Free PMC article.
-
Prevalence, Discontinuation Rate, and Risk Factors for Severe Local Site Reactions with Topical Field Treatment Options for Actinic Keratosis of the Face and Scalp.Medicina (Kaunas). 2019 Apr 4;55(4):92. doi: 10.3390/medicina55040092. Medicina (Kaunas). 2019. PMID: 30987411 Free PMC article. Review.
-
The impact of the current United States guidelines on the management of actinic keratosis: is it time for an update?J Clin Aesthet Dermatol. 2010 Nov;3(11):20-5. J Clin Aesthet Dermatol. 2010. PMID: 21103312 Free PMC article.
-
Refusal of Retreatment With Topical 5-Fluorouracil Among Patients With Actinic Keratosis: Qualitative Analysis.JMIR Dermatol. 2023 Feb 15;6:e39988. doi: 10.2196/39988. JMIR Dermatol. 2023. PMID: 37632916 Free PMC article.
References
-
- Cockerell CJ, Wharton JR. New histopathological classification of actinic keratosis (incipient intraepidermal squamous cell carcinoma) J Drugs Dermatol. 2005;4(4):462–467. - PubMed
-
- Oppel T, Korting HC. Actinic keratosis: the key event in the evolution from photoaged skin to squamous cell carcinoma. Therapy based on pathogenetic and clinical aspects. Skin Pharmacol Physiol. 2004;17(2):67–76. - PubMed
-
- Cockerell CJ. Histopathology of incipient intraepidermal squamous cell carcinoma (“actinic keratosis”) J Am Acad Dermatol. 2000;42(1 Pt 2):11–17. - PubMed
-
- Cockerell CJ. Should we rename actinic keratosis? A leading dermatologist debates a growing dispute in the medical community—the renaming of this precancerous lesion. Skin & Aging. 2002;10(9):31–32.
-
- Glogau RG. The risk of progression to invasive disease. J Am Acad Dermatol. 2000;42(1 Pt 2):S23–S24. - PubMed
LinkOut - more resources
Full Text Sources