A comparative analysis shows morphofunctional differences between the rat and mouse melanin-concentrating hormone systems
- PMID: 21103352
- PMCID: PMC2984507
- DOI: 10.1371/journal.pone.0015471
A comparative analysis shows morphofunctional differences between the rat and mouse melanin-concentrating hormone systems
Abstract
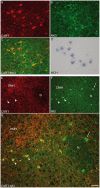
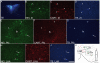
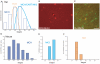
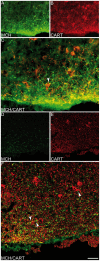
Sub-populations of neurons producing melanin-concentrating hormone (MCH) are characterized by distinct projection patterns, birthdates and CART/NK3 expression in rat. Evidence for such sub-populations has not been reported in other species. However, given that genetically engineered mouse lines are now commonly used as experimental models, a better characterization of the anatomy and morphofunctionnal organization of MCH system in this species is then necessary. Combining multiple immunohistochemistry experiments with in situ hybridization, tract tracing or BrdU injections, evidence supporting the hypothesis that rat and mouse MCH systems are not identical was obtained: sub-populations of MCH neurons also exist in mouse, but their relative abundance is different. Furthermore, divergences in the distribution of MCH axons were observed, in particular in the ventromedial hypothalamus. These differences suggest that rat and mouse MCH neurons are differentially involved in anatomical networks that control feeding and the sleep/wake cycle.
Conflict of interest statement
Figures


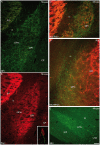

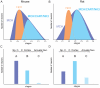
Similar articles
-
Ontogenetic development of the diencephalic MCH neurons: a hypothalamic 'MCH area' hypothesis.Eur J Neurosci. 2001 May;13(9):1733-44. doi: 10.1046/j.0953-816x.2001.01552.x. Eur J Neurosci. 2001. PMID: 11359525
-
Immunocytochemical detection of the neurokinin B receptor (NK3) on melanin-concentrating hormone (MCH) neurons in rat brain.J Chem Neuroanat. 1997 Mar;12(3):183-9. doi: 10.1016/s0891-0618(96)00200-1. J Chem Neuroanat. 1997. PMID: 9141650
-
Optogenetic activation of melanin-concentrating hormone neurons increases non-rapid eye movement and rapid eye movement sleep during the night in rats.Eur J Neurosci. 2016 Nov;44(10):2846-2857. doi: 10.1111/ejn.13410. Epub 2016 Oct 16. Eur J Neurosci. 2016. PMID: 27657541 Free PMC article.
-
The development of the MCH system.Peptides. 2009 Nov;30(11):1969-72. doi: 10.1016/j.peptides.2009.07.016. Epub 2009 Aug 6. Peptides. 2009. PMID: 19647770 Review.
-
Role of the melanin-concentrating hormone neuropeptide in sleep regulation.Peptides. 2009 Nov;30(11):2052-9. doi: 10.1016/j.peptides.2009.07.022. Epub 2009 Aug 4. Peptides. 2009. PMID: 19660508 Review.
Cited by
-
Characterization of Hypothalamic MCH Neuron Development in a 3D Differentiation System of Mouse Embryonic Stem Cells.eNeuro. 2022 Apr 26;9(2):ENEURO.0442-21.2022. doi: 10.1523/ENEURO.0442-21.2022. Print 2022 Mar-Apr. eNeuro. 2022. PMID: 35437265 Free PMC article.
-
Melanin concentrating hormone projections to the nucleus accumbens enhance the reward value of food consumption and do not induce feeding or REM sleep.bioRxiv [Preprint]. 2024 Nov 12:2024.11.11.622987. doi: 10.1101/2024.11.11.622987. bioRxiv. 2024. Update in: J Neurosci. 2025 Mar 05;45(10):e1725242024. doi: 10.1523/JNEUROSCI.1725-24.2024. PMID: 39605522 Free PMC article. Updated. Preprint.
-
Projections from the dorsomedial division of the bed nucleus of the stria terminalis to hypothalamic nuclei in the mouse.J Comp Neurol. 2021 Apr 1;529(5):929-956. doi: 10.1002/cne.24988. Epub 2020 Aug 3. J Comp Neurol. 2021. PMID: 32678476 Free PMC article.
-
Neurokinin B and the control of the gonadotropic axis in the rat: developmental changes, sexual dimorphism, and regulation by gonadal steroids.Endocrinology. 2012 Oct;153(10):4818-29. doi: 10.1210/en.2012-1287. Epub 2012 Jul 20. Endocrinology. 2012. PMID: 22822161 Free PMC article.
-
Molecular profiling of activated neurons by phosphorylated ribosome capture.Cell. 2012 Nov 21;151(5):1126-37. doi: 10.1016/j.cell.2012.10.039. Cell. 2012. PMID: 23178128 Free PMC article.
References
-
- Shimada M, Tritos NA, Lowell BB, Flier JS, Maratos-Flier E. Mice lacking melanin-concentrating hormone are hypophagic and lean. Nature. 1998;396:670–674. - PubMed
-
- Griffond B, Baker BI. Cell and molecular cell biology of melanin-concentrating hormone. Int Rev Cytol. 2002;213:233–277. - PubMed
-
- Hanriot L, Camargo N, Courau AC, Leger L, Luppi PH, et al. Characterization of the melanin-concentrating hormone neurons activated during paradoxical sleep hypersomnia in rats. J Comp Neurol. 2007;505:147–157. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

