Alterations in mGluR5 expression and signaling in Lewy body disease and in transgenic models of alpha-synucleinopathy--implications for excitotoxicity
- PMID: 21103359
- PMCID: PMC2982819
- DOI: 10.1371/journal.pone.0014020
Alterations in mGluR5 expression and signaling in Lewy body disease and in transgenic models of alpha-synucleinopathy--implications for excitotoxicity
Retraction in
-
Retraction: Alterations in mGluR5 Expression and Signaling in Lewy Body Disease and in Transgenic Models of Alpha-Synucleinopathy-Implications for Excitotoxicity.PLoS One. 2024 Nov 14;19(11):e0314138. doi: 10.1371/journal.pone.0314138. eCollection 2024. PLoS One. 2024. PMID: 39541384 Free PMC article. No abstract available.
Abstract
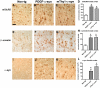
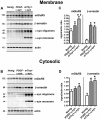
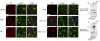
Dementia with Lewy bodies (DLB) and Parkinson's Disease (PD) are neurodegenerative disorders of the aging population characterized by the abnormal accumulation of alpha-synuclein (alpha-syn). Previous studies have suggested that excitotoxicity may contribute to neurodegeneration in these disorders, however the underlying mechanisms and their relationship to alpha-syn remain unclear. For this study we proposed that accumulation of alpha-syn might result in alterations in metabotropic glutamate receptors (mGluR), particularly mGluR5 which has been linked to deficits in murine models of PD. In this context, levels of mGluR5 were analyzed in the brains of PD and DLB human cases and alpha-syn transgenic (tg) mice and compared to age-matched, unimpaired controls, we report a 40% increase in the levels of mGluR5 and beta-arrestin immunoreactivity in the frontal cortex, hippocampus and putamen in DLB cases and in the putamen in PD cases. In the hippocampus, mGluR5 was more abundant in the CA3 region and co-localized with alpha-syn aggregates. Similarly, in the hippocampus and basal ganglia of alpha-syn tg mice, levels of mGluR5 were increased and mGluR5 and alpha-syn were co-localized and co-immunoprecipitated, suggesting that alpha-syn interferes with mGluR5 trafficking. The increased levels of mGluR5 were accompanied by a concomitant increase in the activation of downstream signaling components including ERK, Elk-1 and CREB. Consistent with the increased accumulation of alpha-syn and alterations in mGluR5 in cognitive- and motor-associated brain regions, these mice displayed impaired performance in the water maze and pole test, these behavioral alterations were reversed with the mGluR5 antagonist, MPEP. Taken together the results from study suggest that mGluR5 may directly interact with alpha-syn resulting in its over activation and that this over activation may contribute to excitotoxic cell death in select neuronal regions. These results highlight the therapeutic importance of mGluR5 antagonists in alpha-synucleinopathies.
Conflict of interest statement
Figures

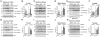



Similar articles
-
Hippocampal neuronal cells that accumulate α-synuclein fragments are more vulnerable to Aβ oligomer toxicity via mGluR5--implications for dementia with Lewy bodies.Mol Neurodegener. 2014 May 19;9:18. doi: 10.1186/1750-1326-9-18. Mol Neurodegener. 2014. Retraction in: Mol Neurodegener. 2025 Aug 5;20(1):87. doi: 10.1186/s13024-025-00881-6. PMID: 24885390 Free PMC article. Retracted.
-
Neurotoxic conversion of beta-synuclein: a novel approach to generate a transgenic mouse model of synucleinopathies?J Neurol. 2009 Aug;256 Suppl 3:286-92. doi: 10.1007/s00415-009-5246-8. J Neurol. 2009. PMID: 19711118 Review.
-
Neuroinflammation is associated with infiltration of T cells in Lewy body disease and α-synuclein transgenic models.J Neuroinflammation. 2020 Jul 17;17(1):214. doi: 10.1186/s12974-020-01888-0. J Neuroinflammation. 2020. PMID: 32680537 Free PMC article.
-
Selective molecular alterations in the autophagy pathway in patients with Lewy body disease and in models of alpha-synucleinopathy.PLoS One. 2010 Feb 19;5(2):e9313. doi: 10.1371/journal.pone.0009313. PLoS One. 2010. Retraction in: PLoS One. 2024 Nov 13;19(11):e0313935. doi: 10.1371/journal.pone.0313935. PMID: 20174468 Free PMC article. Retracted.
-
Formation and development of Lewy pathology: a critical update.J Neurol. 2009 Aug;256 Suppl 3:270-9. doi: 10.1007/s00415-009-5243-y. J Neurol. 2009. PMID: 19711116 Review.
Cited by
-
Targeting β-arrestin2 in the treatment of L-DOPA-induced dyskinesia in Parkinson's disease.Proc Natl Acad Sci U S A. 2015 May 12;112(19):E2517-26. doi: 10.1073/pnas.1502740112. Epub 2015 Apr 27. Proc Natl Acad Sci U S A. 2015. PMID: 25918399 Free PMC article.
-
Group I mGluRs in Therapy and Diagnosis of Parkinson's Disease: Focus on mGluR5 Subtype.Biomedicines. 2022 Apr 7;10(4):864. doi: 10.3390/biomedicines10040864. Biomedicines. 2022. PMID: 35453614 Free PMC article. Review.
-
Metabotropic glutamate receptor 5 inhibits α-synuclein-induced microglia inflammation to protect from neurotoxicity in Parkinson's disease.J Neuroinflammation. 2021 Jan 18;18(1):23. doi: 10.1186/s12974-021-02079-1. J Neuroinflammation. 2021. PMID: 33461598 Free PMC article.
-
Animal models of α-synucleinopathy for Parkinson disease drug development.Nat Rev Neurosci. 2017 Sep;18(9):515-529. doi: 10.1038/nrn.2017.75. Epub 2017 Jul 13. Nat Rev Neurosci. 2017. PMID: 28747776 Review.
-
Characterization of age/sex and the regional distribution of mGluR5 availability in the healthy human brain measured by high-resolution [(11)C]ABP688 PET.Eur J Nucl Med Mol Imaging. 2016 Jan;43(1):152-162. doi: 10.1007/s00259-015-3167-6. Epub 2015 Aug 21. Eur J Nucl Med Mol Imaging. 2016. PMID: 26290423
References
-
- McKeith IG, Dickson DW, Lowe J, Emre M, O'Brien JT, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–1872. - PubMed
-
- Shastry BS. Parkinson disease: etiology, pathogenesis and future of gene therapy. Neurosci Res. 2001;41:5–12. - PubMed
-
- Irizarry M, Growdon W, Gomez-Isla T, Newell K, George J, et al. Nigral and cortical Lewy bodies and dystrophic nigral neurites in Parkinson's disease and cortical Lewy body disease contain alphalpha-synuclein immunoreactivity. JNeuropatholExpNeurol. 1998;57:334–337. - PubMed
-
- Takeda A, Hashimoto M, Mallory M, Sundsmo M, Hansen L, et al. Abnormal distribution of the non-Ab component of Alzheimer's disease amyloid precursor/alpha-synuclein in Lewy body disease as revealed by proteinase K and formic acid pretreatment. LabInvest. 1998;78:1169–1177. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

