Reactive oxygen species are required for maintenance and differentiation of primary lung fibroblasts in idiopathic pulmonary fibrosis
- PMID: 21103368
- PMCID: PMC2982828
- DOI: 10.1371/journal.pone.0014003
Reactive oxygen species are required for maintenance and differentiation of primary lung fibroblasts in idiopathic pulmonary fibrosis
Abstract
Background: Idiopathic pulmonary fibrosis (IPF) is a progressive and fatal illness whose pathogenesis remains poorly understood. Recent evidence suggests oxidative stress as a key player in the establishment/progression of lung fibrosis in animal models and possibly in human IPF. The aim of the present study was to characterize the cellular phenotype of fibroblasts derived from IPF patients and identify underlying molecular mechanisms.
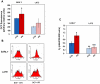
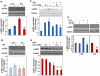
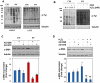
Methodology/principal findings: We first analyzed the baseline differentiation features and growth ability of primary lung fibroblasts derived from 7 histology proven IPF patients and 4 control subjects at different culture passages. Then, we focused on the redox state and related molecular pathways of IPF fibroblasts and investigated the impact of oxidative stress in the establishment of the IPF phenotype. IPF fibroblasts were differentiated into alpha-smooth muscle actin (SMA)-positive myofibroblasts, displayed a pro-fibrotic phenotype as expressing type-I collagen, and proliferated lower than controls cells. The IPF phenotype was inducible upon oxidative stress in control cells and was sensitive to ROS scavenging. IPF fibroblasts also contained large excess of reactive oxygen species (ROS) due to the activation of an NADPH oxidase-like system, displayed higher levels of tyrosine phosphorylated proteins and were more resistant to oxidative-stress induced cell death. Interestingly, the IPF traits disappeared with time in culture, indicating a transient effect of the initial trigger.
Conclusions/significance: Robust expression of α-SMA and type-I collagen, high and uniformly-distributed ROS levels, resistance to oxidative-stress induced cell death and constitutive activation of tyrosine kinase(s) signalling are distinctive features of the IPF phenotype. We suggest that this phenotype can be used as a model to identify the initial trigger of IPF.
Conflict of interest statement
Figures
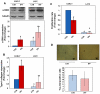
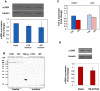
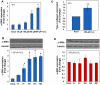
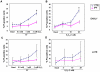

References
-
- American Thoracic Society and European Respiratory Society. American Thoracic Society/European Respiratory Society international multidisciplinary consensus classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2002;165:277–304. - PubMed
-
- King TE, Jr, Schwarz MI, Brown K, Tooze JA, Colby TV, et al. Idiopathic pulmonary fibrosis: relationship between histopathologic features and mortality. Am J Respir Crit Care Med. 2001;164:1025–1032. - PubMed
-
- Nicholson AG, Fulford LG, Colby TV, du Bois RM, Hansell DM, et al. The relationship between individual histologic features and disease progression in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2002;166:173–177. - PubMed
-
- Flaherty KR, Colby TV, Travis WD, Toews GB, Mumford J, et al. Fibroblastic foci in usual interstitial pneumonia. Idiopathic versus collagen vascular disease. Am J Respir Crit Care Med. 2003;167:1410–1415. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

