Automated high-content live animal drug screening using C. elegans expressing the aggregation prone serpin α1-antitrypsin Z
- PMID: 21103396
- PMCID: PMC2980495
- DOI: 10.1371/journal.pone.0015460
Automated high-content live animal drug screening using C. elegans expressing the aggregation prone serpin α1-antitrypsin Z
Abstract
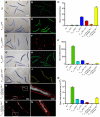
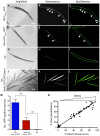
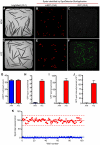
The development of preclinical models amenable to live animal bioactive compound screening is an attractive approach to discovering effective pharmacological therapies for disorders caused by misfolded and aggregation-prone proteins. In general, however, live animal drug screening is labor and resource intensive, and has been hampered by the lack of robust assay designs and high throughput work-flows. Based on their small size, tissue transparency and ease of cultivation, the use of C. elegans should obviate many of the technical impediments associated with live animal drug screening. Moreover, their genetic tractability and accomplished record for providing insights into the molecular and cellular basis of human disease, should make C. elegans an ideal model system for in vivo drug discovery campaigns. The goal of this study was to determine whether C. elegans could be adapted to high-throughput and high-content drug screening strategies analogous to those developed for cell-based systems. Using transgenic animals expressing fluorescently-tagged proteins, we first developed a high-quality, high-throughput work-flow utilizing an automated fluorescence microscopy platform with integrated image acquisition and data analysis modules to qualitatively assess different biological processes including, growth, tissue development, cell viability and autophagy. We next adapted this technology to conduct a small molecule screen and identified compounds that altered the intracellular accumulation of the human aggregation prone mutant that causes liver disease in α1-antitrypsin deficiency. This study provides powerful validation for advancement in preclinical drug discovery campaigns by screening live C. elegans modeling α1-antitrypsin deficiency and other complex disease phenotypes on high-content imaging platforms.
Conflict of interest statement
Figures




References
-
- Aguzzi A, O'Connor T. Protein aggregation diseases: pathogenicity and therapeutic perspectives. Nat Rev Drug Discov. 2010;9:237–248. - PubMed
-
- Herczenik E, Gebbink MF. Molecular and cellular aspects of protein misfolding and disease. FASEB J. 2008;22:2115–2133. - PubMed
-
- Krebs MP, Holden DC, Joshi P, Clark CL, 3rd, Lee AH, et al. Molecular mechanisms of rhodopsin retinitis pigmentosa and the efficacy of pharmacological rescue. J Mol Biol. 2010;395:1063–1078. - PubMed
-
- Lomas DA, Belorgey D, Mallya M, Miranda E, Kinghorn KJ, et al. Molecular mousetraps and the serpinopathies. Biochem Soc Trans. 2005;33:321–330. - PubMed
-
- Picken MM. Amyloidosis-where are we now and where are we heading? Arch Pathol Lab Med. 2010;134:545–551. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

