Viewpoints on Acid-induced inflammatory mediators in esophageal mucosa
- PMID: 21103419
- PMCID: PMC2978390
- DOI: 10.5056/jnm.2010.16.4.374
Viewpoints on Acid-induced inflammatory mediators in esophageal mucosa
Abstract
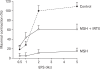
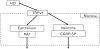
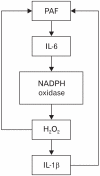
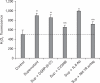
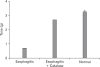
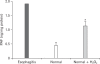
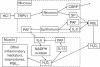
We have focused on understanding the onset of gastroesophageal reflux disease by examining the mucosal response to the presence of acid in the esophageal lumen. Upon exposure to HCl, inflammation of the esophagus begins with activation of the transient receptor potential channel vanilloid subfamily member-1 (TRPV1) in the mucosa, and production of IL-8, substance P (SP), calcitonin gene related peptide (CGRP) and platelet activating factor (PAF). Production of SP and CGRP, but not PAF, is abolished by the neural blocker tetrodotoxin suggesting that SP and CGRP are neurally released and that PAF arises from non neural pathways. Epithelial cells contain TRPV1 receptor mRNA and protein and respond to HCl and to the TRPV1 agonist capsaicin with production of PAF. PAF, SP and IL-8 act as chemokines, inducing migration of peripheral blood leukocytes. PAF and SP activate peripheral blood leukocytes inducing the production of H(2)O(2). In circular muscle, PAF causes production of IL-6, and IL-6 causes production of additional H(2)O(2), through activation of reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidases. Among these, NADPH oxidase 5 cDNA is significantly up-regulated by exposure to PAF; H(2)O(2) content of esophageal and lower esophageal sphincter circular muscle is elevated in human esophagitis, causing dysfunction of esophageal circular muscle contraction and reduction in esophageal sphincter tone. Thus esophageal keratinocytes, that constitute the first barrier to the refluxate, may also serve as the initiating cell type in esophageal inflammation, secreting inflammatory mediators and pro-inflammatory cytokines and affecting leukocyte recruitment and activity.
Keywords: Cytokines; Gastroesophageal reflux disease; Platelet activating factor; Substance P; TRPV1.
Conflict of interest statement
Conflicts of interest: None.
Figures









Similar articles
-
HCl-activated neural and epithelial vanilloid receptors (TRPV1) in cat esophageal mucosa.Am J Physiol Gastrointest Liver Physiol. 2009 Jul;297(1):G135-43. doi: 10.1152/ajpgi.90386.2008. Epub 2009 Apr 23. Am J Physiol Gastrointest Liver Physiol. 2009. PMID: 19389802 Free PMC article.
-
HCl-induced inflammatory mediators in cat esophageal mucosa and inflammatory mediators in esophageal circular muscle in an in vitro model of esophagitis.Am J Physiol Gastrointest Liver Physiol. 2006 Jun;290(6):G1307-17. doi: 10.1152/ajpgi.00576.2005. Epub 2006 Jan 26. Am J Physiol Gastrointest Liver Physiol. 2006. PMID: 16439466
-
HCl-induced and ATP-dependent upregulation of TRPV1 receptor expression and cytokine production by human esophageal epithelial cells.Am J Physiol Gastrointest Liver Physiol. 2012 Sep 1;303(5):G635-45. doi: 10.1152/ajpgi.00097.2012. Epub 2012 Jul 12. Am J Physiol Gastrointest Liver Physiol. 2012. PMID: 22790593 Free PMC article.
-
[Ethiopathogenesis of gastroesophageal reflux disease].Cas Lek Cesk. 2000 Aug 2;139(15):455-9. Cas Lek Cesk. 2000. PMID: 22666924 Review. Czech.
-
Mucosal pathogenesis in gastro-esophageal reflux disease.Neurogastroenterol Motil. 2020 Dec;32(12):e14022. doi: 10.1111/nmo.14022. Epub 2020 Oct 28. Neurogastroenterol Motil. 2020. PMID: 33118247 Review.
Cited by
-
Long-term maintenance effect of radiofrequency energy delivery for refractory GERD: a decade later.Surg Endosc. 2014 Aug;28(8):2323-33. doi: 10.1007/s00464-014-3461-6. Epub 2014 Feb 22. Surg Endosc. 2014. PMID: 24562599
-
Mechanisms of the adenosine A2A receptor-induced sensitization of esophageal C fibers.Am J Physiol Gastrointest Liver Physiol. 2016 Feb 1;310(3):G215-23. doi: 10.1152/ajpgi.00350.2014. Epub 2015 Nov 12. Am J Physiol Gastrointest Liver Physiol. 2016. PMID: 26564719 Free PMC article.
-
Upregulation of the transient receptor potential vanilloid 1 in colonic epithelium of patients with active inflammatory bowel disease.Int J Clin Exp Pathol. 2017 Nov 1;10(11):11335-11344. eCollection 2017. Int J Clin Exp Pathol. 2017. PMID: 31966488 Free PMC article.
-
Distinction between patients with non-erosive reflux disease and functional heartburn.Ann Gastroenterol. 2013;26(4):283-289. Ann Gastroenterol. 2013. PMID: 24714313 Free PMC article. Review.
-
Mucosal neuroimmune mechanisms in gastro-oesophageal reflux disease (GORD) pathogenesis.J Gastroenterol. 2024 Mar;59(3):165-178. doi: 10.1007/s00535-023-02065-9. Epub 2024 Jan 14. J Gastroenterol. 2024. PMID: 38221552 Free PMC article. Review.
References
-
- Fass R. Erosive esophagitis and nonerosive reflux disease (NERD): comparison of epidemiologic, physiologic, and therapeutic characteristics. J Clin Gastroenterol. 2007;41:131–137. - PubMed
-
- Locke GR, 3rd, Talley NJ, Fett SL, Zinsmeister AR, Melton LJ., 3rd Prevalence and clinical spectrum of gastroesophageal reflux: a population-based study in Olmsted County, Minnesota. Gastroenterology. 1997;112:1448–1456. - PubMed
-
- Moayyedi P, Talley NJ. Gastro-oesophageal reflux disease. Lancet. 2006;367:2086–2100. - PubMed
-
- Orlando RC. Reflux esophagitis: overview. Scand J Gastroenterol Suppl. 1995;210:36–37. - PubMed
-
- Orlando RC. Pathophysiology of gastroesophageal reflux disease. J Clin Gastroenterol. 2008;42:584–588. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

