Mechano-energetics of the asynchronous and resynchronized heart
- PMID: 21103927
- PMCID: PMC3074058
- DOI: 10.1007/s10741-010-9205-3
Mechano-energetics of the asynchronous and resynchronized heart
Erratum in
- Heart Fail Rev. 2011 May;16(3):225. Deboeck, Bart W L [corrected to De Boeck, Bart W L]
Abstract
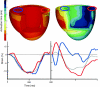
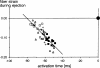
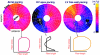
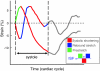
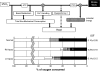
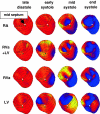
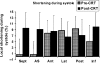
Abnormal electrical activation of the ventricles creates major abnormalities in cardiac mechanics. Local contraction patterns, as reflected by measurements of local strain, are not only out of phase, but often also show opposing length changes in early and late activated regions. As a consequence, the efficiency of cardiac pump function (the amount of stroke work generated by a unit of oxygen consumed) is approximately 30% lower in asynchronous than in synchronous hearts. Moreover, the amount of work performed in myocardial segments becomes considerably larger in late than in early activated regions. Cardiac Resynchronization Therapy (CRT) improves mechano-energetics of the previously asynchronous heart in various ways: it alleviates impediment of the abnormal contraction on blood flow, it increases myocardial efficiency, it recruits contraction in the previously early activated septum and it creates a more uniform distribution of myocardial blood flow. These factors act together to increase the range of cardiac work that can be delivered by the patients' heart, an effect that can explain the increased exercise tolerance and quality of life reported in several CRT trials.
Figures

Similar articles
-
Electro-energetics of Biventricular, Septal and Conduction System Pacing.Arrhythm Electrophysiol Rev. 2021 Dec;10(4):250-257. doi: 10.15420/aer.2021.30. Arrhythm Electrophysiol Rev. 2021. PMID: 35106177 Free PMC article. Review.
-
Timing of myocardial shortening determines left ventricular regional myocardial work and regional remodelling in hearts with conduction delays.Eur Heart J Cardiovasc Imaging. 2018 Aug 1;19(8):941-949. doi: 10.1093/ehjci/jex325. Eur Heart J Cardiovasc Imaging. 2018. PMID: 29272366
-
Mechano-electrical coupling as framework for understanding functional remodeling during LBBB and CRT.Am J Physiol Heart Circ Physiol. 2014 Jun 15;306(12):H1644-59. doi: 10.1152/ajpheart.00689.2013. Epub 2014 Apr 18. Am J Physiol Heart Circ Physiol. 2014. PMID: 24748591
-
Endocardial left ventricular pacing improves cardiac resynchronization therapy in chronic asynchronous infarction and heart failure models.Circ Arrhythm Electrophysiol. 2012 Feb;5(1):191-200. doi: 10.1161/CIRCEP.111.965814. Epub 2011 Nov 7. Circ Arrhythm Electrophysiol. 2012. PMID: 22062796
-
Cardiac Resynchronization Therapy Optimization: A Comprehensive Approach.Cardiology. 2019;142(2):116-128. doi: 10.1159/000499192. Epub 2019 May 22. Cardiology. 2019. PMID: 31117077 Review.
Cited by
-
Considering Diastolic Dyssynchrony as a Predictor of Favorable Response in LV-Only Fusion Pacing Cardiac Resynchronization Therapy.Diagnostics (Basel). 2023 Mar 21;13(6):1186. doi: 10.3390/diagnostics13061186. Diagnostics (Basel). 2023. PMID: 36980494 Free PMC article.
-
Efficiency is key.Eur Heart J Cardiovasc Imaging. 2020 Feb 1;21(2):154-156. doi: 10.1093/ehjci/jez260. Eur Heart J Cardiovasc Imaging. 2020. PMID: 31630186 Free PMC article. No abstract available.
-
Etiologic impact on difference on clinical outcomes of patients with heart failure after cardiac resynchronization therapy: A systematic review and meta-analysis.Medicine (Baltimore). 2018 Dec;97(52):e13725. doi: 10.1097/MD.0000000000013725. Medicine (Baltimore). 2018. PMID: 30593144 Free PMC article.
-
Clinical Long-Term Response to Cardiac Resynchronization Therapy Is Independent of Persisting Echocardiographic Markers of Dyssynchrony.Cardiol Res. 2014 Dec;5(6):163-170. doi: 10.14740/cr368w. Epub 2014 Dec 4. Cardiol Res. 2014. PMID: 28352448 Free PMC article.
-
Pilot study using 3D-longitudinal strain computation in a multi-parametric approach for best selecting responders to cardiac resynchronization therapy.Cardiovasc Ultrasound. 2017 Jun 17;15(1):15. doi: 10.1186/s12947-017-0107-6. Cardiovasc Ultrasound. 2017. PMID: 28623910 Free PMC article.
References
-
- Auricchio A, Stellbrink C, Block M, Sack S, Vogt J, Bakker P, Klein H, Kramer A, Ding J, Salo R, Tockman B, Pochet T, Spinelli J. Effect of pacing chamber and atrioventricular delay on acute systolic function of paced patients with congestive heart failure. The pacing therapies for congestive heart failure study group. The guidant congestive heart failure research group. Circulation. 1999;99:2993–3001. - PubMed
-
- Badke FR, Boinay P, Covell JW. Effect of ventricular pacing on regional left ventricular performance in the dog. Am J Physiol. 1980;238:H858–H867. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

