Quantification of particle residence time in abdominal aortic aneurysms using magnetic resonance imaging and computational fluid dynamics
- PMID: 21103933
- PMCID: PMC3066149
- DOI: 10.1007/s10439-010-0202-4
Quantification of particle residence time in abdominal aortic aneurysms using magnetic resonance imaging and computational fluid dynamics
Abstract
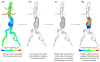
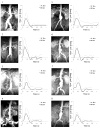
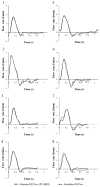
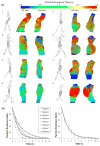
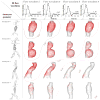
Hemodynamic conditions are hypothesized to affect the initiation, growth, and rupture of abdominal aortic aneurysms (AAAs), a vascular disease characterized by progressive wall degradation and enlargement of the abdominal aorta. This study aims to use magnetic resonance imaging (MRI) and computational fluid dynamics (CFD) to quantify flow stagnation and recirculation in eight AAAs by computing particle residence time (PRT). Specifically, we used gadolinium-enhanced MR angiography to obtain images of the vessel lumens, which were used to generate subject-specific models. We also used phase-contrast MRI to measure blood flow at supraceliac and infrarenal locations to prescribe physiologic boundary conditions. CFD was used to simulate pulsatile flow, and PRT, particle residence index, and particle half-life of PRT in the aneurysms were computed. We observed significant regional differences of PRT in the aneurysms with localized patterns that differed depending on aneurysm geometry and infrarenal flow. A bulbous aneurysm with the lowest mean infrarenal flow demonstrated the slowest particle clearance. In addition, improvements in particle clearance were observed with increase of mean infrarenal flow. We postulate that augmentation of mean infrarenal flow during exercise may reduce chronic flow stasis that may influence mural thrombus burden, degradation of the vessel wall, and aneurysm growth.
Figures





References
-
- Bluestein D, Niu L, Schoephoerster RT, Dewanjee MK. Steady flow in an aneurysm model: correlation between fluid dynamics and blood platelet deposition. J Biomech Eng. 1996;118:280–286. - PubMed
-
- Bluestein D, Niu L, Schoephoerster RT, Dewanjee MK. Fluid mechanics of arterial stenosis: relationship to the development of mural thrombus. Ann Biomed Eng. 1997;25(2):344–356. - PubMed
-
- Butty VD, Gudjonsson K, Buchel P, Makhijani VB, Ventikos Y, Poulikakos D. Residence times and basins of attraction for a realistic right internal carotid artery with two aneurysms. Biorheology. 2002;29:387–393. - PubMed
-
- Cao J, Rittgers SE. Particle motion within in vitro models of stenosed internal carotid and left anterior descending coronary arteries. Ann Biomed Eng. 1998;26:190– 199. - PubMed
-
- Cheng CP, Herfkens RJ, Taylor CA. Abdominal aortic hemodynamic conditions in healthy subjects aged 50–70 at rest and during lower limb exercise: in vivo quantification using MRI. Atherosclerosis. 2003;168:323–331. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

