Role of the intestinal bile acid transporters in bile acid and drug disposition
- PMID: 21103970
- PMCID: PMC3249407
- DOI: 10.1007/978-3-642-14541-4_4
Role of the intestinal bile acid transporters in bile acid and drug disposition
Abstract
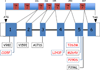
Membrane transporters expressed by the hepatocyte and enterocyte play critical roles in maintaining the enterohepatic circulation of bile acids, an effective recycling and conservation mechanism that largely restricts these potentially cytotoxic detergents to the intestinal and hepatobiliary compartments. In doing so, the hepatic and enterocyte transport systems ensure a continuous supply of bile acids to be used repeatedly during the digestion of multiple meals throughout the day. Absorption of bile acids from the intestinal lumen and export into the portal circulation is mediated by a series of transporters expressed on the enterocyte apical and basolateral membranes. The ileal apical sodium-dependent bile acid cotransporter (abbreviated ASBT; gene symbol, SLC10A2) is responsible for the initial uptake of bile acids across the enterocyte brush border membrane. The bile acids are then efficiently shuttled across the cell and exported across the basolateral membrane by the heteromeric Organic Solute Transporter, OSTα-OSTβ. This chapter briefly reviews the tissue expression, physiology, genetics, pathophysiology, and transport properties of the ASBT and OSTα-OSTβ. In addition, the chapter discusses the relationship between the intestinal bile acid transporters and drug metabolism, including development of ASBT inhibitors as novel hypocholesterolemic or hepatoprotective agents, prodrug targeting of the ASBT to increase oral bioavailability, and involvement of the intestinal bile acid transporters in drug absorption and drug-drug interactions.
Figures

References
-
- The Lipid Research Clinics Coronary Primary Prevention Trial results. I. Reduction in incidence of coronary heart disease. JAMA. 1984;251:351–364. - PubMed
-
- Aldini R, Montagnani M, Roda A, Hrelia S, Biagi PL, Roda E. Intestinal absorption of bile acids in the rabbit: different transport rates in jejunum and ileum. Gastroenterology. 1996;110:459–468. - PubMed
-
- Aldini R, Roda A, Montagnani M, Polimeni C, Lenzi PL, Cerre C, Galletti G, Roda E. Hepatic uptake and intestinal absorption of bile acids in the rabbit. Eur J Clin Invest. 1994;24:691–697. - PubMed
-
- Alpini G, Glaser S, Baiocchi L, Francis H, Xia X, Lesage G. Secretin activation of the apical Na+-dependent bile acid transporter is associated with cholehepatic shunting in rats. Hepatology. 2005;41:1037–1045. - PubMed
-
- Alpini G, Glaser SS, Rodgers R, Phinizy JL, Robertson WE, Lasater J, Caligiuri A, Tretjak Z, LeSage GD. Functional expression of the apical Na+-dependent bile acid transporter in large but not small rat cholangiocytes. Gastroenterology. 1997a;113:1734–1740. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

