Removal and reconstitution of the carotenoid antenna of xanthorhodopsin
- PMID: 21104180
- PMCID: PMC3030941
- DOI: 10.1007/s00232-010-9322-x
Removal and reconstitution of the carotenoid antenna of xanthorhodopsin
Abstract
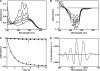
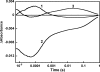
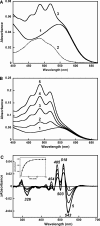
Salinixanthin, a C(40)-carotenoid acyl glycoside, serves as a light-harvesting antenna in the retinal-based proton pump xanthorhodopsin of Salinibacter ruber. In the crystallographic structure of this protein, the conjugated chain of salinixanthin is located at the protein-lipid boundary and interacts with residues of helices E and F. Its ring, with a 4-keto group, is rotated relative to the plane of the π-system of the carotenoid polyene chain and immobilized in a binding site near the β-ionone retinal ring. We show here that the carotenoid can be removed by oxidation with ammonium persulfate, with little effect on the other chromophore, retinal. The characteristic CD bands attributed to bound salinixanthin are now absent. The kinetics of the photocycle is only slightly perturbed, showing a 1.5-fold decrease in the overall turnover rate. The carotenoid-free protein can be reconstituted with salinixanthin extracted from the cell membrane of S. ruber. Reconstitution is accompanied by restoration of the characteristic vibronic structure of the absorption spectrum of the antenna carotenoid, its chirality, and the excited-state energy transfer to the retinal. Minor modification of salinixanthin, by reducing the carbonyl C=O double bond in the ring to a C-OH, suppresses its binding to the protein and eliminates the antenna function. This indicates that the presence of the 4-keto group is critical for carotenoid binding and efficient energy transfer.
Figures





Similar articles
-
Femtosecond carotenoid to retinal energy transfer in xanthorhodopsin.Biophys J. 2009 Mar 18;96(6):2268-77. doi: 10.1016/j.bpj.2009.01.004. Biophys J. 2009. PMID: 19289053 Free PMC article.
-
Induced chirality of the light-harvesting carotenoid salinixanthin and its interaction with the retinal of xanthorhodopsin.Biochemistry. 2006 Sep 12;45(36):10998-1004. doi: 10.1021/bi061098i. Biochemistry. 2006. PMID: 16953586 Free PMC article.
-
Reconstitution of Gloeobacter violaceus rhodopsin with a light-harvesting carotenoid antenna.Biochemistry. 2009 Nov 24;48(46):10948-55. doi: 10.1021/bi901552x. Biochemistry. 2009. PMID: 19842712 Free PMC article.
-
Xanthorhodopsin: Proton pump with a carotenoid antenna.Cell Mol Life Sci. 2007 Sep;64(18):2323-8. doi: 10.1007/s00018-007-7167-y. Cell Mol Life Sci. 2007. PMID: 17571211 Free PMC article. Review.
-
Salinibacter: an extremely halophilic bacterium with archaeal properties.FEMS Microbiol Lett. 2013 May;342(1):1-9. doi: 10.1111/1574-6968.12094. Epub 2013 Feb 25. FEMS Microbiol Lett. 2013. PMID: 23373661 Review.
Cited by
-
Expression of Xanthorhodopsin in Escherichia coli.Protein J. 2023 Aug;42(4):408-420. doi: 10.1007/s10930-023-10109-5. Epub 2023 Mar 31. Protein J. 2023. PMID: 37002449
-
Characterization of an Unconventional Rhodopsin from the Freshwater Actinobacterium Rhodoluna lacicola.J Bacteriol. 2015 Aug;197(16):2704-12. doi: 10.1128/JB.00386-15. Epub 2015 Jun 8. J Bacteriol. 2015. PMID: 26055118 Free PMC article.
-
Rhodopsins: An Excitingly Versatile Protein Species for Research, Development and Creative Engineering.Front Chem. 2022 Jun 22;10:879609. doi: 10.3389/fchem.2022.879609. eCollection 2022. Front Chem. 2022. PMID: 35815212 Free PMC article. Review.
-
Selective Choice of the Efficient Carotenoid Antenna by a Xanthorhodopsin: Controlling Factors for Binding and Excitation Energy Transfer.JACS Au. 2025 Jun 26;5(7):3070-3081. doi: 10.1021/jacsau.4c01243. eCollection 2025 Jul 28. JACS Au. 2025. PMID: 40747033 Free PMC article.
-
Carotenoids bind rhodopsins and act as photocycle-accelerating pigments in marine Bacteroidota.Nat Microbiol. 2025 Sep 4. doi: 10.1038/s41564-025-02109-1. Online ahead of print. Nat Microbiol. 2025. PMID: 40908369
References
-
- Antón J, Oren A, Benlloch S, et al. Salinibacter ruber gen. nov., sp. nov., a novel, extremely halophilic member of the Bacteria from saltern crystallizer ponds. Int J Syst Evol Micrbiol. 2002;52:485–491. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

