Modulation of E-cadherin function and dysfunction by N-glycosylation
- PMID: 21104290
- PMCID: PMC11114786
- DOI: 10.1007/s00018-010-0595-0
Modulation of E-cadherin function and dysfunction by N-glycosylation
Abstract
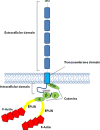
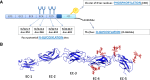
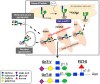
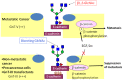
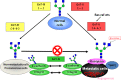
Several mechanisms have been proposed to explain the E-cadherin dysfunction in cancer, including genetic and epigenetic alterations. Nevertheless, a significant number of human carcinomas have been seen that show E-cadherin dysfunction that cannot be explained at the genetic/epigenetic level. A substantial body of evidence has appeared recently that supports the view that other mechanisms operating at the post-translational level may also affect E-cadherin function. The present review addresses molecular aspects related to E-cadherin N-glycosylation and evidence is presented showing that the modification of N-linked glycans on E-cadherin can affect the adhesive function of this adhesion molecule. The role of glycosyltransferases involved in the remodeling of N-glycans on E-cadherin, including N-acetylglucosaminyltransferase III (GnT-III), N-acetylglucosaminyltransferase V (GnT-V), and the α1,6 fucosyltransferase (FUT8) enzyme, is also discussed. Finally, this review discusses an alternative functional regulatory mechanism for E-cadherin operating at the post-translational level, N-glycosylation, that may underlie the E-cadherin dysfunction in some carcinomas.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

