Dipyrimidine amines: a novel class of chemokine receptor type 4 antagonists with high specificity
- PMID: 21105715
- PMCID: PMC3003753
- DOI: 10.1021/jm100786g
Dipyrimidine amines: a novel class of chemokine receptor type 4 antagonists with high specificity
Abstract
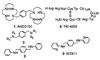
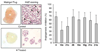
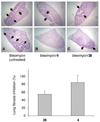
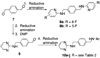
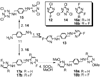
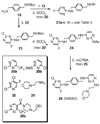
The C-X-C chemokine receptor type 4 (CXCR4)/stromal cell derived factor-1 (SDF-1 or CXCL12) interaction and the resulting cell signaling cascade play a key role in metastasis and inflammation. On the basis of the previously published CXCR4 antagonist 5 (WZ811), a series of novel nonpeptidic anti-CXCR4 small molecules have been designed and synthesized to improve potency. Following a structure-activity profile around 5, more advanced compounds in the N,N'-(1, 4-phenylenebis(methylene)) dipyrimidin-2-amines series were discovered and shown to possess higher CXCR4 binding potential and specificity than 5. Compound 26 (508MCl) is the lead compound and exhibits subnanomolar potency in three in vitro assays including competitive binding, Matrigel invasion and Gα(i) cyclic adenosine monophosphate (cAMP) modulation signaling. Furthermore, compound 26 displays promising effects by interfering with CXCR4 function in three mouse models: paw inflammation, Matrigel plug angiogenesis, and uveal melanoma micrometastasis. These data demonstrate that dipyrimidine amines are unique CXCR4 antagonists with high potency and specificity.
Figures



References
-
- Butcher EC, Williams M, Youngman K, Rott L, Briskin M. Lymphocyte trafficking and regional immunity. Adv Immunol. 1999;72:209–253. - PubMed
-
- Forster R, Schubel A, Breitfeld D, Kremmer E, Renner-Muller I, Wolf E, Lipp M. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23–33. - PubMed
-
- Peled A, Petit I, Kollet O, Magid M, Ponomaryov T, Byk T, Nagler A, Ben-Hur H, Many A, Shultz L, Lider O, Alon R, Zipori D, Lapidot T. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283:845–848. - PubMed
-
- Zaitseva M, Blauvelt A, Lee S, Lapham CK, Klaus-Kovtun V, Mostowski H, Manischewitz J, Golding H. Expression and function of CCR5 and CXCR4 on human Langerhans cells and macrophages: implications for HIV primary infection. Nat Med. 1997;3:1369–1375. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

