Tunable bifunctional silyl ether cross-linkers for the design of acid-sensitive biomaterials
- PMID: 21105720
- PMCID: PMC3097112
- DOI: 10.1021/ja108568g
Tunable bifunctional silyl ether cross-linkers for the design of acid-sensitive biomaterials
Abstract
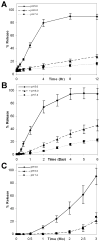
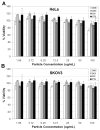
Responsive polymeric biomaterials can be triggered to degrade using localized environments found in vivo. A limited number of biomaterials provide precise control over the rate of degradation and the release rate of entrapped cargo and yield a material that is intrinsically nontoxic. In this work, we designed nontoxic acid-sensitive biomaterials based on silyl ether chemistry. A host of silyl ether cross-linkers were synthesized and molded into relevant medical devices, including Trojan horse particles, sutures, and stents. The resulting devices were engineered to degrade under acidic conditions known to exist in tumor tissue, inflammatory tissue, and diseased cells. The implementation of silyl ether chemistry gave precise control over the rate of degradation and afforded devices that could degrade over the course of hours, days, weeks, or months, depending upon the steric bulk around the silicon atom. These novel materials could be useful for numerous biomedical applications, including drug delivery, tissue repair, and general surgery.
Figures




References
-
- Wuts PGM, Greene TW. Greene’s Protective Groups in Organic Synthesis. 4. Wiley; New York: 2007.
-
- Jones DP, Carlson JL, Mody VC, Cai JY, Lynn MJ, Sternberg P. Free Radical Biology and Medicine. 2000;28:625–635. - PubMed
-
- Meister A. Pharmacology & Therapeutics. 1991;51:155–194. - PubMed
-
- Chau Y, Tan FE, Langer R. Bioconjugate Chemistry. 2004;15:931–941. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

